Dear Editors,
Oxaliplatin is generally classified as an irritant anticancer agent. In certain instances of extravasation, it can provoke severe local reactions such as inflammation, induration, and skin necrosis, suggesting vesicant properties [1, 2]. Rare extravasations through a totally implantable venous access device (TIVAD) can lead to extensive tissue exposure and subsequent injury. Conservative or surgical approaches have been reported for oxaliplatin extravasation through a TIVAD [1–5], but none have yet been established as standard [5]. Herein, we report a case in which massive oxaliplatin extravasation through a TIVAD was successfully managed with early systemic corticosteroid therapy. The patient gave consent for her photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
A 76-year-old woman (body weight, 30 kg) with transverse colon cancer was treated with a modified FOLFOX6 regimen (5-fluorouracil, leucovorin, and oxaliplatin). As part of palliative care, dexamethasone 1.65 mg/day was administered routinely. On day 1 of the third cycle, the patient received oral aprepitant and intravenous dexamethasone 4.95 mg as premedication. Oxaliplatin (75 mg) in 5% glucose solution (250 mL) and leucovorin (220 mg) in 250 mL normal saline were administered via a left chest TIVAD. Complete extravasation of both solutions was observed at the end of the infusion. The patient developed extensive edema and pain in the left chest and back (Figure 1A). Computed tomography (CT) revealed widespread subcutaneous edema in the affected area (Figure 1B).
FIGURE 1
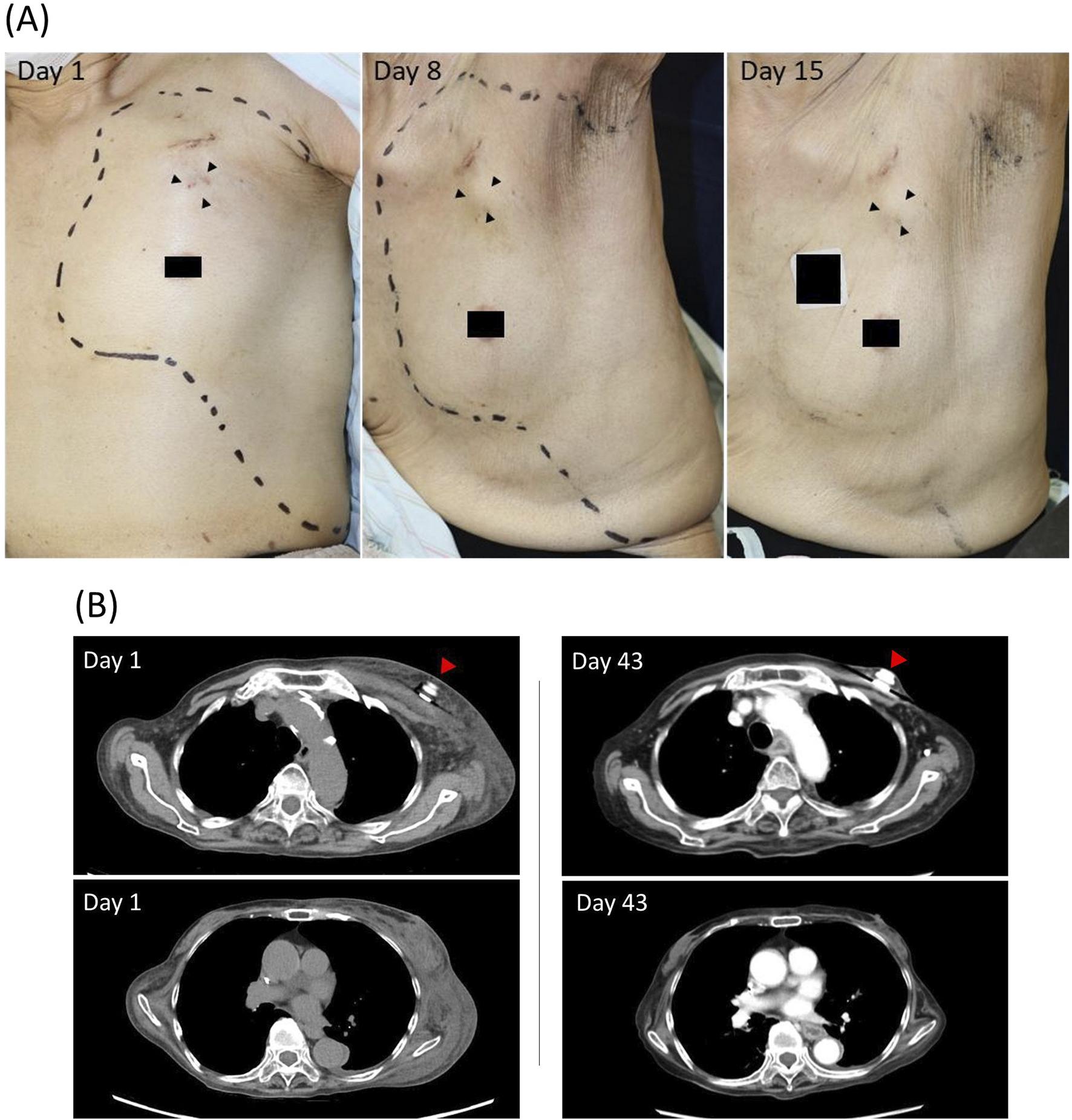
(A) Time-course of clinical presentation in the oxaliplatin-extravasated area. The area of extravasation is indicated by the dotted line. Arrowheads, the site where a totally implantable venous access device is implanted. (B) Computed tomography (CT) findings of the oxaliplatin-extravasated area. On day 1, widespread subcutaneous edema is revealed in the left chest and back, without evidence of mediastinal leakage or damage to the implantable venous access device. On day 43, follow-up CT images revealed no residual of the subcutaneous abnormality. Arrowhead, the totally implantable venous access device.
Conservative treatment was initiated on the same day and consisted of intravenous dexamethasone 4.95 mg/day for 4 days, oral celecoxib 100 mg twice daily, and topical clobetasol propionate. By day 2, the swelling around the TIVAD subsided, although induration with moderate pain persisted in the left lower chest and back. By day 3, the edema in the affected area had completely resolved, with only mild residual discomfort. Dexamethasone was tapered to 3.3 mg on day 5 and further to 1.65 mg (baseline palliative dose) on day 7. Celecoxib and topical corticosteroids were discontinued on days 8 and 10, respectively. Follow-up CT images taken on day 43 revealed no residual of the subcutaneous abnormality due to the oxaliplatin extravasation (Figure 1B). No progression to persistent skin inflammation, necrosis, or other alterations was observed during the 9-week follow-up period.
The literature describes various approaches for oxaliplatin extravasation through a TIVAD, including surgical options such as early wash-out and debridement. Haslik et al. reported successful outcomes with immediate subcutaneous wash-out procedures [4], while Van Praet et al. documented complete resolution following surgical excision of necrotic tissue in a case in which conservative management failed over 9 months [5]. Despite the effectiveness of surgical interventions in selected cases, these procedures are highly invasive.
In contrast, the present case demonstrates that conservative management with early systemic corticosteroids can achieve complete resolutions even after massive oxaliplatin extravasation. Several reports have supported the use of corticosteroids for oxaliplatin extravasation [1, 2, 5], although specific doses and durations have not been established. Kretzschmar A et al. reported dexamethasone 8 mg bid for several days had a positive influence in two patients [1]. In the present case, intravenous dexamethasone (equivalent to prednisolone 1 mg/kg/day for the patient), tapered over 7 days, effectively prevented inflammation and necrosis. Therefore, an immediate course of high-dose systemic corticosteroids for several days may be a rational and effective treatment strategy. Surgical intervention should be considered if persistent local inflammation or necrosis develops.
The decision to administer corticosteroids either locally or systemically should be made based on an evaluation of the pros and cons of each approach. While the topical application and/or local injection of corticosteroids are commonly employed to manage anticancer drug extravasation, the extensive nature of the oxaliplatin extravasation in the present case made local injections across the entire affected area impractical. Moreover, CT images (Figure 1B) showed that the extravasated oxaliplatin had infiltrated at least into the deep adipose tissue, where topical formulations would be unlikely to have a therapeutic effect. Therefore, systemic corticosteroids were selected as the mainstay of treatment, with topical corticosteroids as an additional therapy. In cases involving extensive and deep extravasation, systemic administration may be a more effective and rational approach than local application.
This study has several limitations. First, the relatively low dose or concentration of extravasated oxaliplatin (75 mg), corresponding to the patient’s body weight, might have contributed to the favorable outcome. Although the dose is within the range reported in the previous cases of oxaliplatin extravasation (40–165 mg) [2], it cannot be ruled out the possibility that our intervention might not have caused the rapid resolution of the patient’s symptoms. Secondly, the patient had been receiving a low maintenance dose of dexamethasone for palliative care prior to the event. This corticosteroid use might have dampened the local inflammatory reactivity at baseline.
In conclusion, this case demonstrates that even massive oxaliplatin extravasation through a central venous port can be successfully managed with early conservative therapy with systemic corticosteroids.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because the manuscript is a case report that contains no identifiable information. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ST, MK, and HS contributed to data collection and made an interpretation of the results. ST wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Abbreviations
CT, Computed tomography; TIVAD, totally implantable venous access device.
References
1.
KretzschmarAPinkDThuss-PatiencePDorkenBReichertPEckertR. Extravasations of oxaliplatin. J Clin Oncol (2003) 21:4068–9. 10.1200/JCO.2003.99.095
2.
PericayCLopezASolerJRBonfillTDotorESaigiE. Extravasation of oxaliplatin: an infrequent and irritant toxicity. Clin Transl Oncol (2009) 11:114–6. 10.1007/s12094-009-0324-z
3.
de LemosMLWalisserS. Management of extravasation of oxaliplatin. J Oncol Pharm Pract (2005) 11:159–62. 10.1191/1078155205jp165oa
4.
HaslikWHackerSFelberbauerFXThallingerCBartschRKornauthCet alPort-a-Cath extravasation of vesicant cytotoxics: surgical options for a rare complication of cancer chemotherapy. Eur J Surg Oncol (2015) 41:378–85. 10.1016/j.ejso.2014.11.042
5.
Van PraetLBoecxstaensVDouchyT. Surgical management after Oxaliplatin extravasation: a case report and literature review. J Vasc Access (2023) 24:1239–43. 10.1177/11297298221075237
Summary
Keywords
extravasation, implantable venous access device, corticosteroid, oxaliplatin, surgery
Citation
Takahagi S, Kanamoto M and Sakamoto H (2025) Successful conservative management of massive oxaliplatin extravasation from a central venous port with early systemic corticosteroid therapy. J. Cutan. Immunol. Allergy 8:15049. doi: 10.3389/jcia.2025.15049
Received
07 June 2025
Accepted
08 July 2025
Published
14 July 2025
Volume
8 - 2025
Updates
Copyright
© 2025 Takahagi, Kanamoto and Sakamoto.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shunsuke Takahagi, takshuns@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.