Dear Editors,
Prostate cancer is the second most commonly diagnosed malignancy in men worldwide [1, 2]. For patients with castration-resistant prostate cancer, androgen deprivation therapy combined with second-generation androgen receptor inhibitors, such as apalutamide, has demonstrated improved disease progression and survival rates [2]. A dose-dependent rash is a common adverse event [3], while lichenoid drug eruption (LDE) has been rarely reported [4].
We present the case of a 78-year-old male (body mass index: 30.6) with a history of hypertension managed with amlodipine for 3 years. One prior year, he was diagnosed with prostate cancer (cT4N1M1) and subsequently started on oral apalutamide (240 mg/day). Apalutamide was the first hormone treatment for his life. One month after initiating apalutamide therapy, he developed a rash on his upper limbs, which was asymptomatic. Despite topical corticosteroid treatment at a local clinic, the rash progressed. Six months after initiating apalutamide (5 months after rash onset), he was referred to our department. Clinical examination revealed erythematous plaques with keratotic scales extending to the dorsum of the hands, trunk, and lower limbs (Figures 1a–c). The rash is not present on the mucous membranes and covers approximately 30% of the body surface area. Histopathological analysis showed band-like lymphocytic infiltration, including eosinophils and melanophage, in the papillary dermis. Additionally, the basement membrane exhibited liquefactive degeneration with partial epidermal projections (Figures 1d,e). During administration of apalutamide, the cancer showed a tendency to shrink. Considering the drug rash, a screening test for infectious lesions was conducted before starting systemic prednisone. This test revealed pneumonia. According to the respiratory medicine specialist, this pneumonia could not be ruled out as being caused by infection. During the screening, apalutamide was continued at a reduced dose, but the skin rash worsened, leading to its discontinuation. The rash subsided (Figures 1e,f) a few months later, leading to a diagnosis of grade 3 drug-induced rash (amlodipine was being continued).
FIGURE 1
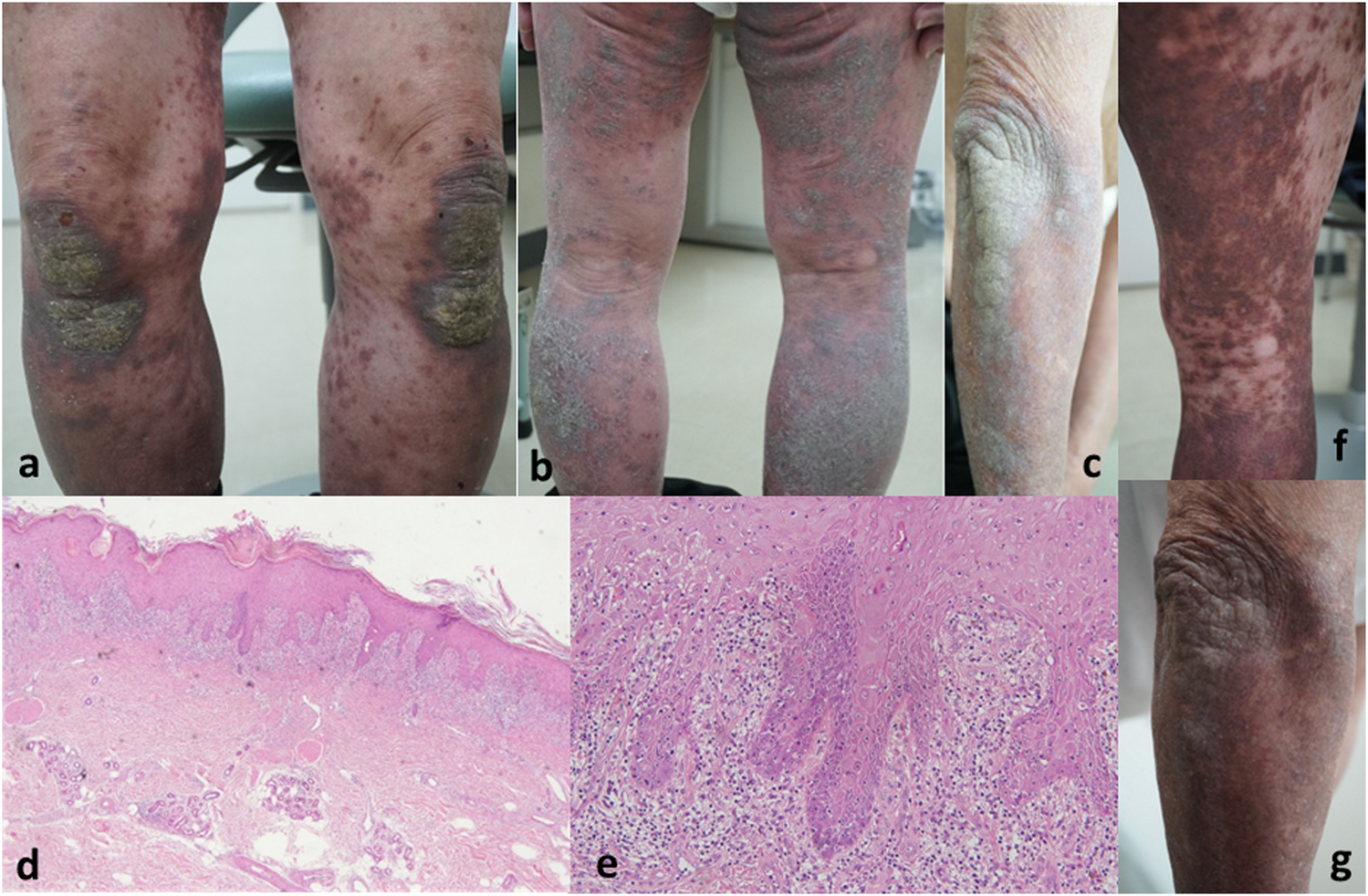
Dark purple–brown erythema with keratotic scaling was observed on multiple body regions. (a) Knees and lower legs. (b) Right forearm. (c) Histopathological findings: Band-like lymphocytic infiltration, including eosinophils and melanophages, in the papillary dermis. Liquefactive degeneration of the basement membrane with partial epidermal projections [(d) ×200 magnification and (e) ×400 magnification] The keratotic scaling of the rash has disappeared, and the erythema has turned into pigmentation. (f) Right lower legs. (g) Right forearm.
Recently, multiple reports have described apalutamide-induced LDE. Prontskus et al. [4] analyzed 16 previously documented cases, demonstrating a significant reporting odds ratio and information component for LDE associated with apalutamide. While LDE remains an uncommon presentation among apalutamide-induced rashes, these findings suggest a non-incidental association between the drug and lichenified eruptions. Standard management includes discontinuation of apalutamide and corticosteroid therapy. In a previous case series [4], the drug was discontinued in all cases where the condition improved.
Grade 3 apalutamide-induced rashes typically manifest within 4 months of treatment initiation. However, previous reports have documented LDE cases with extensive rash development occurring after a prolonged latency (over 12 months) [1], consistent with the gradual evolution of lichenoid lesions [1, 4]. This trend is the same for LDEs by other drugs. In contrast, our case is notable for its early onset, occurring just 1 month after apalutamide initiation. In addition, other few cases of apalutamide-induced LDE within 1 month were present [4]. No definitive evidence supports a shorter incubation period in our patient. A major limitation of our case is the inability to assess the patient’s skin condition before or during the early rash stage. Notably, drug eruptions associated with amlodipine have also been reported [5], raising the possibility of a multifactorial etiology involving drug interactions or predisposing conditions.
Given the limited data available, LDE can potentially occur at any stage of apalutamide therapy. Moreover, there have been a few reported severe LDE cases that developed shortly after starting apalutamide [4], as in this case. Therefore, clinicians should remain vigilant for drug-induced eruptions when evaluating new or worsening skin rashes, irrespective of treatment duration.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conceptualization, SH and KI; methodology, SY; validation, CW; formal analysis, CW; investigation, CW; writing—original draft preparation, SH; writing—review and editing, KI. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
GuglielminiPFMassoneCGrassoCFranceseAVincentiMChiodiSet alA rare and severe lichenoid skin eruption after apalutamide treatment for prostate cancer. Ann Dermatol Venereol (2023) 15:310–1. 10.1016/j.annder.2023.09.001
2.
SaadFBögemannMSuzukiKShoreN. Treatment of nonmetastatic castrationresistant prostate cancer: focus on second-generation androgen receptor inhibitors. Prostate Cancer Prostatic Dis (2021) 24:323–34. 10.1038/s41391-020-00310-3
3.
EndoYOkaAUeharaATokiSMotegiSIIshikawaOet alFatal case of toxic epidermal necrolysis due to apalutamide used as a novel prostate cancer drug. J Dermatol (2020) 47:e359–60. 10.1111/1346-8138.15510
4.
ProntskusVPinaultALScailteuxLMBeyensMNFresseAPetitpainN. Lichenoid drug eruption and apalutamide: analyses from pharmacovigilance databases and disproportionality analysis. Therapie (2025) (25), S0040–5957. 10.1016/j.therap.2024.12.013
5.
Muhammad HafizMPAzidahAKZainabMY. Amlodipine-induced buccal lichenoid lesions: a case report, Malays Fam Physician (2024) 19:17, 10.51866/cr.531
Summary
Keywords
adverse drug reaction, lichenified skin rash, apalutamide, drug eruption, prostate cancer
Citation
Watanabe C, Hayashi S, Tokoro S and Igawa K (2025) Severe lichenified skin rash associated with apalutamide administration. J. Cutan. Immunol. Allergy 8:14936. doi: 10.3389/jcia.2025.14936
Received
21 May 2025
Accepted
09 July 2025
Published
22 July 2025
Volume
8 - 2025
Updates
Copyright
© 2025 Watanabe, Hayashi, Tokoro and Igawa.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shujiro Hayashi, shayashi@dokkyomed.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.