Dear Editors,
Immune checkpoint inhibitors (ICIs) have emerged as promising treatment options for various malignancies. However, their use is often associated with severe immune-related adverse events (irAEs), including Stevens–Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN), affecting approximately 1%–2% of ICI-treated patients [
1]. Although ophthalmic irAEs have been reported in around 1% of cases, the clinical manifestations of severe ophthalmic irAEs associated with SJS/TEN remain poorly understood [
1,
2]. Here, we present two cases experiencing irAE-SJS with severe ocular complications, evaluated using the SJS ophthalmologic severity score [
3].
Case 1
A 69-year-old man with stage IV renal cell carcinoma developed mild eyelid erythema and conjunctival hyperemia a few days after initiating nivolumab and ipilimumab in July 201X. After completing three courses of immunotherapy, he was referred to our department in September 201X, because his condition worsened, presenting with tense facial bullae and mucosal erosions on the cheek with a positive Nikolsky sign (
Figures 1a,b). Ophthalmological examinations revealed conjunctival hyperemia, edema, pseudomembrane formation, and discharge (
Figure 1c). Histopathological examination of a skin biopsy obtained from the forehead, where the erosive erythema with a positive Nikolsky sign was observed (
Figure 1b), revealed a lichenoid reaction, intraepidermal and subepidermal bullae, and perivascular infiltration of lymphocytes, histiocytes, and eosinophils (
Figure 1d). Direct immunofluorescence (DIF) tests of the basement membrane zone and intercellular space were negative (data not shown), and his blood tested negative for anti-desmograin 1 and 3 and anti-BP180 antibodies. Based on these findings, we ruled out autoimmune blistering diseases. The patient was concomitantly taking amlodipine and doxazosin, which tested negative in a drug-induced lymphocyte stimulation test (DLST) while on oral prednisolone (PSL) at a dose of 20 mg/day. Testing for infections such as
Mycoplasmaand herpes simplex virus (HSV) was not performed. Thus, we diagnosed the condition as an irAE-SJS. After immediate discontinuation of ICI therapy and concomitant drugs. He was treated with systemic corticosteroids (PSL 1 mg/kg/day), ophthalmic pseudomembrane debridement, antibiotics, immunosuppressive eye drops, and subconjunctival dexamethasone injections. Despite significant improvement in the skin rash, ocular symptoms persisted. Ophthalmologic severity scoring revealed severe epithelial defects, loss of palisades of Vogt, neovascularization, and hyperemia (grade 3) (
Figures 1e, m) [
3]. Despite maintaining PSL at 10 mg/day, the patient experienced significant visual loss 6 months after discontinuing immunotherapy. During treatment for the irAE-SJS, he was at high risk of rupture of an abdominal aortic aneurysm, and steroid pulse therapy was considered a critical risk factor for elevated blood pressure; its rupture caused sudden death.
Case 2
FIGURE 1
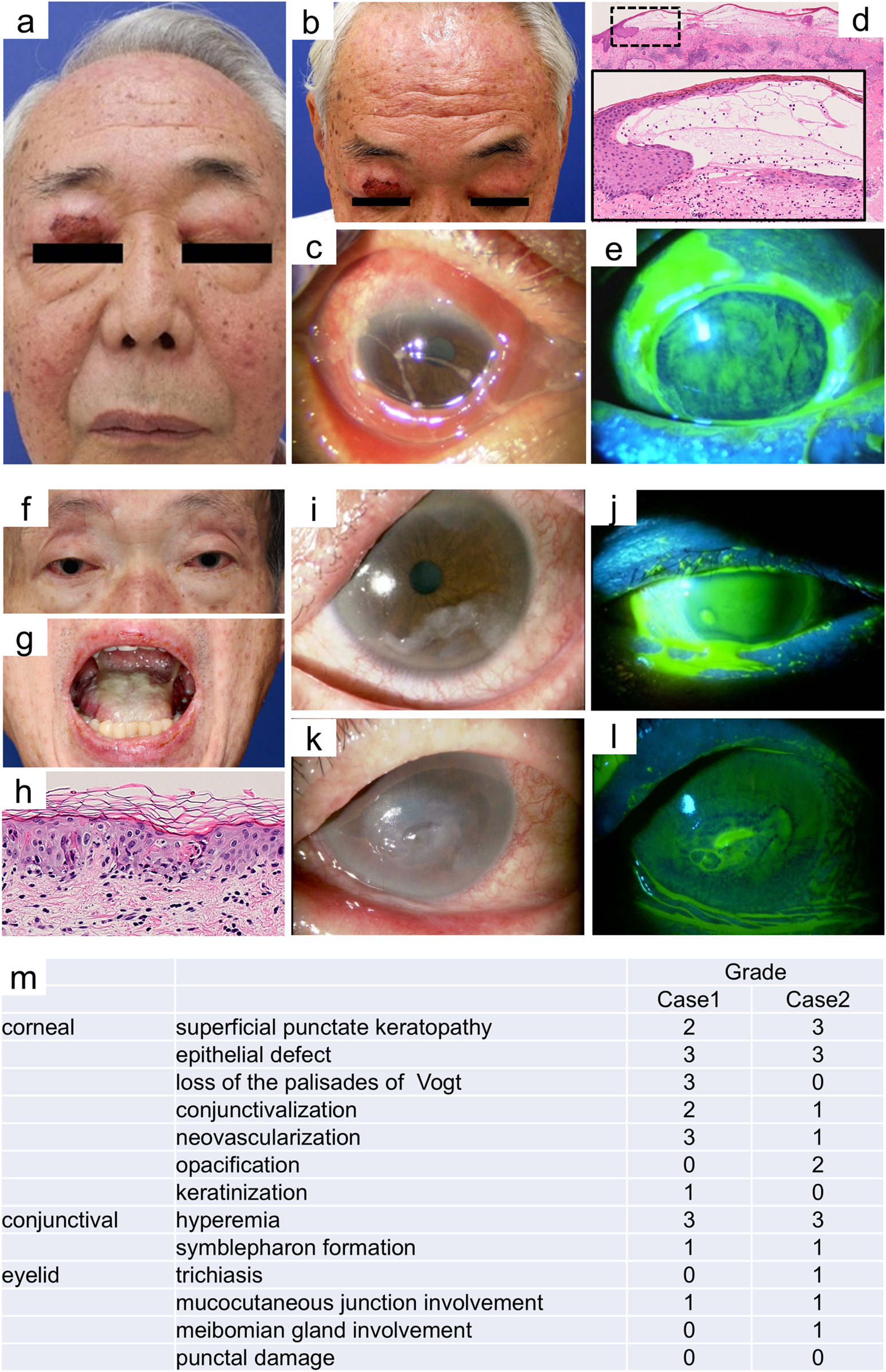
Case 1: (a) At initial presentation, after three courses of immune checkpoint inhibitor (ICI) therapy, the patient exhibited bilateral eyelid edema with erosion and crusting and mild epistaxis. In addition, conjunctival hyperemia and large amounts of viscous eye discharge were observed. (b) Clinical presentation included the biopsy obtained from the forehead. (c) After resolution of the conjunctival edema, the patient developed bilateral symblepharon formation, significantly limiting ocular motility and vision. (d) Histopathological examination of a skin biopsy taken from the forehead revealed a lichenoid reaction, intraepidermal and subepidermal bullae and perivascular infiltration of lymphocytes, histiocytes, and eosinophils (hematoxylin and eosin stain, original magnification ×25). The black dotted area is enlarged in the inset (original magnification ×200). (e) Fluorescein staining revealed conjunctival erosion in the left eye 4 months after the discontinuation of immune ICI therapy. Case 2: (f) At initial presentation, the patient exhibited bilateral eyelid edema and conjunctival hyperemia. (g) In addition, oral erosions were observed. (h) Histopathological examination of skin biopsy of the back revealed epidermal necrosis, subepidermal bullae, interface dermatitis, and infiltration of lymphocytes and histiocytes into the dermal papillae and perivascular areas (hematoxylin and eosin stain, original magnification ×300). (i, j) Ocular examination revealed marked conjunctival hyperemia, edema, and superficial punctate keratopathy. (k, l) Persistent corneal erosions were observed in the left eye 4 months after diagnosis of irAE-SJS. (m) Ophthalmologic severity scores for Cases 1 and 2 are shown. Note: Blurring has been applied to the iris in Panel (f) to protect patient identity.
A 64-year-old man with a history of esophagogastric junction cancer developed irAE-dermatomyositis after completing seven courses of combination therapy with oxaliplatin, tegafur–gimeracil–oteracil potassium (TS-1), and nivolumab in April 202Y. He discontinued ICI therapy and underwent steroid pulse therapy, followed by oral PSL at 40 mg/day with sulfamethoxazole–trimethoprim (SMT-TMP) in May 202Y. Despite discontinuing ICI therapy and receiving PSL at 30 mg/day, he developed fever, conjunctival hyperemia, oral erosions, and a diffuse erythematous rash with a positive Nikolsky sign approximately on June 20, 202Y (Figures 1f–j). A skin biopsy taken from the back revealed epidermal necrosis, subepidermal bullae, interface dermatitis, and infiltration of lymphocytes and histiocytes into the dermal papillae and perivascular areas (Figure 1h). DIF tests of the basement membrane zone and intercellular space were negative (data not shown). Several concomitant medications, including SMT-TMP and metformin hydrochloride, which were potential causative agents, were immediately discontinued. All drugs tested negative in DLSTs while on PSL at a dose of 5 mg/day. His blood tested negative for anti-desmograin 1 and 3 and anti-BP180 antibodies. Cytomegalovirus, HSV-1, and HSV-2 antigens were negative. We diagnosed the condition as an irAE-SJS, and the patient was treated with steroid pulse therapy on June 22, 202Y, followed by systemic corticosteroids (PSL 1 mg/kg/day), ophthalmic pseudomembrane debridement, and antibiotic and immunosuppressive eye drops. Although the skin rash improved significantly, ocular symptoms persisted. Ophthalmologic severity scoring revealed severe epithelial defects, superficial punctate keratopathy (SPK), and hyperemia (grade 3) (Figures 1k–m) [3]. The patient experienced significant visual loss in September 202Y.
In Case 1, the total ophthalmologic severity score for SJS was 19, with symblepharon formation leading to hand motion vision. Case 2 had a score of 17, with residual dry eye and SPK in the left eye causing significant visual loss. Despite the rapid improvement in the skin rash with standard treatments for irAE-SJS, ocular symptoms persisted for >6 months, resulting in severe corneal damage and visual loss. This prolonged ocular involvement may be attributable to the sustained effects of ICI therapy, even after discontinuation [4].
According to the SJS/TEN treatment algorithm, steroid pulse therapy is recommended for patients with severe ocular involvement, specifically for those with an ocular score ≥2 [5]. Furthermore, in patients with SJS who develop corneal damage, immediate administration of steroid pulse therapy in addition to high-dose systemic steroids (PSL at 1 mg/kg/day) should be considered because it may help prevent future visual loss [5]. Because of complications (unruptured abdominal aortic aneurysm), steroid pulse therapy could not be administered in Case 1. In contrast, although steroid pulse therapy was appropriately initiated 2 days after the onset of irAE-SJS in Case 2, ocular involvement progressed.
Typical SJS is a severe skin and mucous membrane disorder characterized by erythema, erosions, and blisters. Histological findings of SJS include full-thickness epidermal necrosis with subepidermal blister formation [3, 5]. On the other hand, skin and mucosal symptoms associated with irAEs during ICI therapy are often diverse. To differentiate these symptoms from SJS, it is essential to consider autoimmune blistering disorders such as bullous pemphigoid, pemphigus vulgaris, and paraneoplastic pemphigus. We described two cases in which patients undergoing ICI therapy developed erythema and erosions on the skin, along with ocular symptoms and oral mucosal erosions. After excluding autoimmune blistering diseases, we diagnosed these conditions as irAE-SJS.
In conclusion, close collaboration between dermatologists and ophthalmologists is essential to optimize the management and prognosis of ocular complications associated with ICI therapy.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
EsenBHÖzbekLOğuzSSelçukbiricikF. Characterizing immune checkpoint inhibitor-related cutaneous adverse reactions: a comprehensive analysis of FDA adverse event reporting system (FAERS) database. Heliyon (2024) 10(13):e33765. 10.1016/j.heliyon.2024.e33765
2.
DalvinLAShieldsCLOrloffMSatoTShieldsJA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina (2018) 38(6):1063–78. 10.1097/IAE.0000000000002181
3.
SotozonoCAngLPKoizumiNHigashiharaHUetaMInatomiTet alNew grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology (2007) 114(7):1294–302. 10.1016/j.ophtha.2006.10.029
4.
TopalianSLSznolMMcDermottDFKlugerHMCarvajalRDSharfmanWHet alSurvival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol (2014) 32(10):1020–30. 10.1200/JCO.2013.53.0105
5.
ArakiYSotozonoCInatomiTUetaMYokoiNUedaEet alSuccessful treatment of Stevens-Johnson syndrome with steroid pulse therapy at disease onset. Am J Ophthalmol (2009) 147(6):1004–11. 10.1016/j.ajo.2008.12.040
Summary
Keywords
immune-related adverse event, nivolumab, ipilimumab, Stevens-Johnson syndrome, symblepharon
Citation
Nakahara-Omori Y, Kobayashi Y, Ishizaki S, Chujo K, Suto C, Tanaka M and Umegaki-Arao N (2025) Two cases of Stevens-Johnson syndrome presenting severe ocular complications associated with immune checkpoint inhibitor. J. Cutan. Immunol. Allergy 8:14843. doi: 10.3389/jcia.2025.14843
Received
01 May 2025
Accepted
27 June 2025
Published
10 July 2025
Volume
8 - 2025
Updates
Copyright
© 2025 Nakahara-Omori, Kobayashi, Ishizaki, Chujo, Suto, Tanaka and Umegaki-Arao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noriko Umegaki-Arao, umegaki.noriko@twmu.ac.jp
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.