Abstract
Background:
Dupilumab, a human anti-interleukin-4 receptor α monoclonal antibody, was approved in Japan in 2018 as an add-on to topical therapy for atopic dermatitis (AD) inadequately controlled with conventional therapies in patients aged ≥15 years. A regulatory-mandated post-marketing surveillance (PMS) of the real-world safety and effectiveness of dupilumab was conducted.
Methods:
Patients with AD who initiated dupilumab treatment between July 2018 and June 2020 in Japan were monitored for 2 years. Safety outcomes included adverse drug reactions (ADRs) and some adverse events (AEs). Effectiveness outcomes included changes in the Eczema Area and Severity Index (EASI), Investigator’s Global Assessment (IGA), and pruritus numerical rating scale (NRS) scores.
Results:
Of the 989 patients registered from 184 sites, 962 and 892 were included in the safety and effectiveness analysis sets, respectively. At 2 years, the incidence rates of any ADR and serious ADRs (16.8% and 0.8%, respectively) were similar to those at the 1-year interim analysis. The most common ADRs were conjunctivitis (7.1%), allergic conjunctivitis (4.2%), and blepharitis (0.8%). For effectiveness at 2 years, significant improvements from baseline were observed for mean ± standard deviation EASI (from 30.1 ± 13.2 to 4.0 ± 7.0), IGA (from 3.4 ± 0.5 to 1.4 ± 0.8), and weekly peak pruritus NRS (from 6.9 ± 2.1 to 1.8 ± 1.6) scores (all P < 0.001 vs. baseline).
Limitations:
The observational, non-comparative design precludes establishing causality, with concomitant AD treatments potentially confounding dupilumab’s effectiveness assessment and non-compulsory follow-up visits may have introduced selection bias.
Conclusion:
The real-world PMS in Japan confirmed that dupilumab was well-tolerated, demonstrated an acceptable safety profile and was associated with sustained improvements in AD signs and symptoms over 2 years.
Trial Registration:
UMIN-CTR (https://www.umin.ac.jp/ctr/index.htm), Identifier: UMIN000032807.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory systemic disease characterized by signs of xerosis (dry skin), eczematous lesions, and pruritus [1–3]. The pathophysiology of AD is complex and involves interactions between genetic and environmental factors, type 2 inflammatory mechanisms, and impairment in the epidermal barrier and skin microbiome [1, 4].
Japanese clinical practice guidelines for AD recommend first-line topical anti-inflammatory agents (e.g., topical corticosteroids, tacrolimus, delgocitinib) to control pruritus and inflammation, in combination with regular skin care (e.g., topical moisturizers) to rehydrate and restore the barrier function of the skin [2]. In patients unable to achieve AD remission with topical therapies, systemic treatments such as cyclosporin, dupilumab, and Janus kinase inhibitors, as well as phototherapy and a psychological approach to managing AD are recommended to achieve remission, while oral antihistamines are recommended as an adjunct treatment for pruritus [2]. Once remission is achieved with dupilumab, the guidelines recommend dupilumab for long-term maintenance therapy [2]. Recently newer biologics therapeutic agents (nemolizumab, tralokinumab, and lebrikizumab) have been developed and became available [5].
Dupilumab is a human monoclonal antibody that targets the shared interleukin (IL)-4 receptor α subunit of the IL-4 and IL-13 receptors [6, 7]. In 2018, dupilumab was approved in Japan for the treatment of AD not adequately controlled with conventional therapies as an add-on to topical therapy for patients aged ≥15 years [2]. Following the approval of dupilumab for the treatment of AD in Japan, a 2-year post-marketing surveillance (PMS) was initiated to monitor its real-world safety and effectiveness in current Japanese clinical practice [8]. Herein we report the final 2-year results, which aimed to evaluate the real-world, long-term safety and effectiveness of dupilumab in patients with moderate-to-severe AD in Japan.
Materials and methods
Post-marketing surveillance design
The design and 1-year interim results of this PMS have been previously described [8]. Briefly, this observational, multicenter PMS (UMIN Clinical Trials Registry ID, UMIN000032807) enrolled individuals who first received dupilumab for the treatment of moderate-to-severe AD in Japanese clinical practice. For reimbursement in Japan, dupilumab is recommended for individuals not adequately controlled after ≥6 months of conventional therapies (i.e., topical anti-inflammatory medications), with Eczema Area and Severity Index (EASI) score ≥16 (or EASI head and neck sub score ≥2.4), Investigator’s Global Assessment (IGA) score ≥3 (i.e., moderate-to-severe disease), and AD lesions on ≥10% of the total body surface area [2]. The approved dosage of dupilumab for the treatment of AD in adults is 600 mg administered by subcutaneous injection for the first dose, followed by 300 mg subcutaneous injections every 2 weeks thereafter [2].
Patients were registered into the PMS between July 2018 and June 2020 (registration period) and monitored for a maximum of 2 years following dupilumab initiation (observation period).
This PMS was conducted in accordance with Japanese Ministerial Ordinance on Good Post-marketing Study Practice and the Fair Competition Code in Ethical Pharmaceutical Drugs Marketing Industry; ethics approval for the study was obtained from participating sites, and all patients provided voluntary informed consent. Only data from the sites that permitted publication of the data were included in the present analysis.
Outcome measures
Data describing patient characteristics and the safety and effectiveness of dupilumab were obtained from electronic case report forms (CRFs) collected throughout the observation period [8]. To understand the real-world usage of dupilumab, treatment discontinuation was broadly defined as either treatment interruption (treatment stopped temporarily and restarted at a later date), deferral (treatment postponed to a later date) or true discontinuation (stopped permanently due to loss of follow-up, for reasons such as change to another hospital or death) of treatment during the observation period, at the discretion of the physicians. Two-year safety outcomes reported in this final PMS analysis include the incidence of adverse drug reactions (ADRs; defined as adverse events [AEs] for which a causal relationship to dupilumab could not be ruled out), and AEs of interest (AEIs).
AEIs are specified in the Japanese Risk Management Plan (J-RMP) for dupilumab as serious hypersensitivity reactions, serious infections, the exacerbation of symptoms associated with other allergic diseases, events associated with depression and suicidal behavior, and malignant tumors [9]. ADRs and AEs were coded using the system organ class and preferred terms according to the Japanese version of Medical Dictionary for Regulatory Activities Terminology (MedDRA/J, version 25.1). Serious events were defined based on the impact on the patient’s health, and an event is considered serious if it results in any of the following: death, life-threatening, hospitalization, disability or incapacity, congenital anomaly/birth defect, or other important medical events.
The effectiveness of dupilumab for AD was evaluated through changes in disease severity, symptoms, biomarkers, and patient-reported outcomes throughout the observation period. Disease severity outcomes included EASI, IGA, and weekly peak pruritus numerical rating scale (NRS) scores measured at baseline and at months 1, 2, 4, 6, 8, 10, 12, 18, and 24.
Additional analyses evaluated the proportion of patients with ≥50%, ≥75%, and ≥90% improvements in the EASI score from baseline (EASI-50, EASI-75, and EASI-90, respectively) over time, and the proportion of patients who achieved an IGA score ≤1 and an IGA score reduction of ≥2 at any time during the observation period. Biomarker data were collected if available, including peripheral eosinophil count and serum levels of lactate dehydrogenase (LDH), C-C motif chemokine ligand (CCL)-17/thymus and activation-regulated chemokine (TARC), and total immunoglobulin E (IgE) measured during the observation period.
Patient-reported outcomes were collected through Dermatology Life Quality Index (DLQI), EuroQol (EQ)-5D, and Work Productivity and Activity Impairment Questionnaire - atopic dermatitis (WPAI-AD) questionnaires, which were conducted at baseline, 4 months, 12 months, and after treatment discontinuation (where evaluable).
Statistical analysis
As previously described, a target sample size of 900 patients was estimated for the safety analysis set [8], which included all registered individuals who had CRFs collected, received at least one dose of dupilumab, and had data available for safety evaluation. Baseline characteristics, prior and concomitant treatments for AD, ADRs, AEIs, and treatment discontinuation during the observation period were summarized using descriptive statistics, including the means, standard deviations (SDs), medians, and/or interquartile ranges (IQRs) for continuous variables, and patient numbers and proportions for categorical variables. Kaplan–Meier analyses were additionally performed to estimate the cumulative incidence [±95% confidence interval (CI)] of ADRs over time. The effectiveness analysis set was defined as all patients in the safety analysis set, excluding those with unknown medication status, a primary disease other than AD, and no effectiveness data available for evaluation. Measures of disease severity, symptoms, biomarkers, and patient-reported outcomes over time were summarized using descriptive statistics and compared with baseline values using t tests. The proportion of patients who achieved an IGA score ≤1 and an IGA score reduction of ≥2 at any time during the observation period (herein referred to as the effective proportion) was evaluated in patients with a baseline IGA score ≥2. The effective proportion was estimated in the overall population and in subgroups stratified by baseline factors; exploratory comparisons between subgroups were performed using Fisher’s exact tests or Cochran-Armitage tests as applicable. Statistical analyses were based on observed data with no imputation for missing values and were performed using SAS version 9.1 or later (SAS Institute Inc., Cary, NC).
Results
Study population
Between July 2018 and June 2020, 989 individuals with moderate-to-severe AD who initiated treatment with dupilumab were registered in this PMS from 184 clinical sites in Japan. Electronic CRFs were collected from 970 patients at 179 sites; after exclusions, the safety analysis set comprised 962 patients, and 892 of those were included in the effectiveness analysis set (Supplementary Figure S1). The median duration of active dupilumab treatment during the observation period was 62.3 weeks (IQR, 22.4–100.3). The median treatment interval of dupilumab, determined as the number of days since the most recent treatment at 6, 12, 18, and 24 months, was 15 days over the 2-year observation period (Supplementary Table S1).
In the safety analysis set, 679 patients (70.6%) were male, and most patients were adults (5.1% were adolescent patients aged 15–18 years; Table 1). Approximately half of the safety analysis set (527 patients; 54.8%) had one or more comorbid allergic diseases at baseline; the most common were allergic rhinitis (194 patients; 20.2%), allergic conjunctivitis (139 patients; 14.4%), and asthma (104 patients; 10.8%). Among those with available data, the EASI score (mean ± SD) was 30.1 ± 13.2 at baseline, and in line with clinical practice guidelines [2]; most patients had IGA score 3 (460/862 patients; 53.4%) and IGA score 4 (388/862 patients; 45.0%) at dupilumab initiation.
TABLE 1
| Demographic and clinical characteristics | Safety analysis set (N = 962) |
|---|---|
| Sex, n (%) | |
| Male | 679 (70.6) |
| Female | 283 (29.4) |
| Age (years), mean ± SD | 41.4 ± 15.7 |
| ≥15 to <18 years, n (%) | 49 (5.1) |
| ≥18 to <65 years, n (%) | 823 (85.6) |
| ≥65 years, n (%) | 90 (9.4) |
| Age at AD onset (years), mean ± SD | 14.7 ± 18.1 |
| <6 years, n (%) | 323 (33.6) |
| ≥6 to <18 years, n (%) | 170 (17.7) |
| ≥18 years, n (%) | 200 (20.8) |
| Unknown, n (%) | 269 (28.0) |
| Duration of AD (years), mean ± SD | 26.3 ± 14.2 |
| <10 years, n (%) | 80 (8.3) |
| ≥10 years, n (%) | 613 (63.7) |
| Unknown, n (%) | 269 (28.0) |
| Comorbid allergic diseases, n (%)a | 527 (54.8) |
| Allergic rhinitis | 194 (20.2) |
| Allergic conjunctivitis | 139 (14.4) |
| Asthma | 104 (10.8) |
| Food allergy | 85 (8.8) |
| EASI score, mean ± SDb | 30.1 ± 13.2 |
| IGA score, mean ± SDc | 3.4 ± 0.5 |
| IGA0 (clear), n (%) | 0 |
| IGA1 (almost clear), n (%) | 2 (0.2) |
| IGA2 (mild), n (%) | 12 (1.4) |
| IGA3 (moderate), n (%) | 460 (53.4) |
| IGA4 (severe), n (%) | 388 (45.0) |
| Weekly peak pruritus NRS score, mean ± SDd | 6.9 ± 2.1 |
| Peripheral eosinophil count (cells/mm3), mean ± SDe | 754.9 ± 765.9 |
| Serum LDH (IU/L), mean ± SDf | 302.2 ± 117.5 |
| Serum TARC (pg/mL), mean ± SDg | 5,011.3 ± 7,397.4 |
| Serum total IgE (IU/mL), mean ± SDh | 10,479.0 ± 11,836.4 |
Baseline patient demographics and clinical characteristics.
Not all allergic comorbidities are shown.
Data are based on 838 patients.
Data are based on 862 patients.
Data are based on 387 patients.
Data are based on 614 patients.
Data are based on 609 patients.
Data are based on 625 patients.
Data are based on 601 patients.
AD, atopic dermatitis; EASI, eczema area and severity index; IG, Investigator’s Global Assessment; IgE, immunoglobulin E; LDH, lactate dehydrogenase; NRS, numerical rating scale; TARC, thymus and activation-regulated chemokine; SD, standard deviation.
Concomitant treatments for AD
Consistent with the clinical practice guidelines, in which dupilumab is recommended as an add-on therapy to topical therapy in Japan [2], almost all patients in this PMS (951 patients; 98.9%) had previously received treatment for AD, and most (942 patients; 97.9%) continued concomitant treatment while receiving dupilumab (Table 2). The most common prior treatments for AD were topical corticosteroids (924 patients; 96.0%), moisturizers (727 patients; 75.6%), and topical calcineurin inhibitors (473 patients; 49.2%). The proportions of patients who received these treatments during the observation period (i.e., in combination with dupilumab) were similar (Table 2).
TABLE 2
| Treatment for AD, n (%)a | Safety analysis set (N = 962) | ||
|---|---|---|---|
| Prior treatmentb | Concomitant treatment | ||
| At baselinec | During the observation period | ||
| Any medication for AD | 951 (98.9) | 939 (97.6) | 942 (97.9) |
| Topical corticosteroids | 924 (96.0) | 892 (92.7) | 898 (93.3) |
| Moisturizers | 727 (75.6) | 714 (74.2) | 717 (74.5) |
| Topical calcineurin inhibitors | 473 (49.2) | 455 (47.3) | 462 (48.0) |
| Oral non-steroidal immunosuppressant | 158 (16.4) | 93 (9.7) | 95 (9.9) |
| Overlapping transition to dupilumab | − | 75 (7.8) | |
| Oral corticosteroids | 104 (10.8) | 60 (6.2) | 64 (6.7) |
| Overlapping transition to dupilumab | − | 46 (4.8) | |
| Others | 623 (64.8) | 585 (60.8) | 588 (61.1) |
| Other treatment for AD | |||
| Ultraviolet phototherapy | 59 (6.1) | 13 (1.4) | 14 (1.5) |
| Inpatient treatment | 46 (4.8) | 7 (0.7) | 7 (0.7) |
| Psychotherapy | 3 (0.3) | 1 (0.1) | 1 (0.1) |
Treatments received prior to, or in combination with, dupilumab.
Patients who received >1 prior or concomitant treatment for AD, are counted in multiple rows.
Defined as treatment received within 3 months before baseline.
The first day of dupilumab treatment.
AD, atopic dermatitis.
Of those who reported prior treatment with oral non-steroidal immunosuppressants (158 patients; 16.4%) and oral corticosteroids (104 patients; 10.8%), 93 (9.7%) and 60 patients (6.2%), respectively, were receiving these treatments at baseline. Of these, 75 patients (7.8%) on baseline oral non-steroidal immunosuppressants and 46 patients (4.8%) on baseline oral corticosteroids reported a period of overlapping administration of these drugs after starting dupilumab treatment. The proportion of patients receiving these treatments decreased throughout the 2-year observation period (Supplementary Figure S2).
Safety outcomes
Over 2 years, ADRs were reported in 162 patients (16.8%), including eight patients (0.8%) with serious ADRs (Table 3; Supplementary Table S2). The most common ADRs were conjunctivitis [68 patients (7.1%), including one (0.1%) with a serious ADR], allergic conjunctivitis [40 patients (4.2%), including one (0.1%) with a serious ADR], and blepharitis [eight patients (0.8%), with no serious ADRs].
TABLE 3
| Type of ADR | Safety analysis set (N = 962) | |
|---|---|---|
| Any ADR, n (%) | Serious ADR, n (%) | |
| Total ADRs | 162 (16.8) | 8 (0.8) |
| Infections and infestations | 73 (7.6) | 3 (0.3) |
| Conjunctivitis | 68 (7.1) | 1 (0.1) |
| Eczema herpeticum | 2 (0.2) | 2 (0.2) |
| Nervous system disorders | 8 (0.8) | 2 (0.2) |
| Headache | 4 (0.4) | 0 |
| Dizziness | 2 (0.2) | 0 |
| Eye disorders | 57 (5.9) | 1 (0.1) |
| Allergic conjunctivitis | 40 (4.2) | 1 (0.1) |
| Blepharitis | 8 (0.8) | 0 |
| Ocular pruritus | 5 (0.5) | 0 |
| Conjunctival hyperemia | 3 (0.3) | 0 |
| Dry eye | 2 (0.2) | 0 |
| Ocular hyperemia | 2 (0.2) | 0 |
| Skin and subcutaneous tissue disorders | 18 (1.9) | 0 |
| Alopecia | 3 (0.3) | 0 |
| Erythema | 3 (0.3) | 0 |
| Acne | 2 (0.2) | 0 |
| Pruritus | 2 (0.2) | 0 |
| General disorders and administration site conditions | 11 (1.1) | 1 (0.1) |
| Pyrexia | 6 (0.6) | 1 (0.1) |
| Injection site erythema | 2 (0.2) | 0 |
| Injection site pain | 2 (0.2) | 0 |
| Injection site pruritus | 2 (0.2) | 0 |
| Investigations | 8 (0.8) | 1 (0.1) |
| Eosinophil count increased | 6 (0.6) | 0 |
Adverse drug reactions that occurred in ≥2 patients during the observation period.
Multiple occurrences of the same ADR in one individual are counted only once; ADRs that results in death are included; ADRs that occurred in <2 patients are listed in Supplementary Table S1.
ADR, adverse drug reaction.
When the incidence of ADRs was assessed by time of onset during the observation period, most events occurred within 120 days of initiating dupilumab (Figures 1, 2). AEIs specified in the J-RMP, regardless of causal relationship with dupilumab, are summarized in Supplementary Table S3. Serious hypersensitivity reactions were reported in four patients (0.4%; all were serious AEIs), serious infections in 11 patients (1.1%; all were serious AEIs), exacerbations of other allergic diseases in 10 patients [1.0%, including one (0.1%) with a serious AEI; eight of these patients (0.8%) experienced an exacerbation of asthma symptoms (Supplementary Table S4) and all eight patients had a history of asthma or comorbid asthma], events associated with depression and suicidal behavior in two patients [0.2%, including one (0.1%) with a serious AEI], and malignant tumors in three patients (0.3%; all were serious AEIs).
FIGURE 1
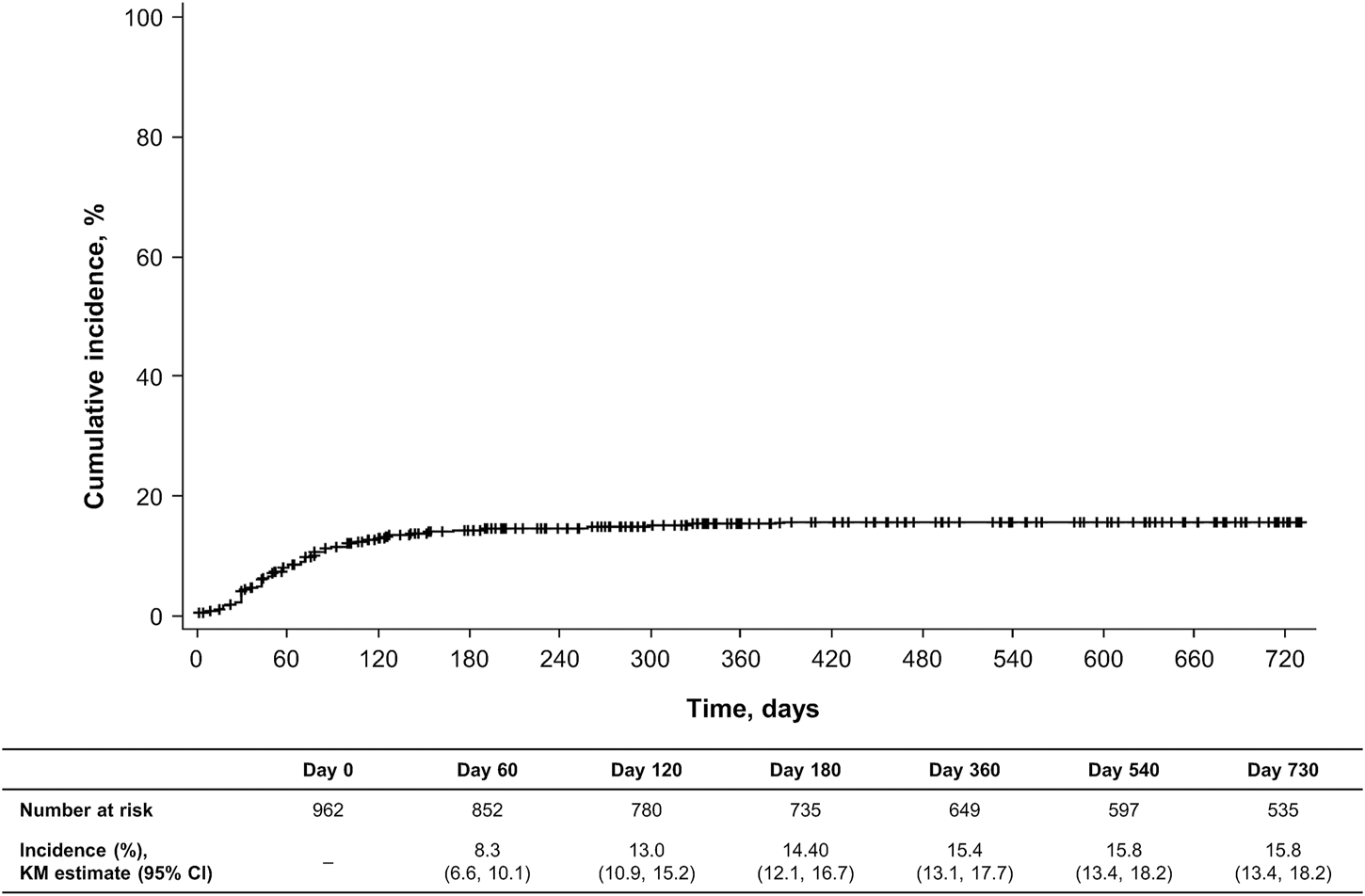
Cumulative incidence of adverse drug reactions during the observation period. Data are based on the safety analysis set (N = 962). CI, confidence interval; KM, Kaplan–Meier.
FIGURE 2
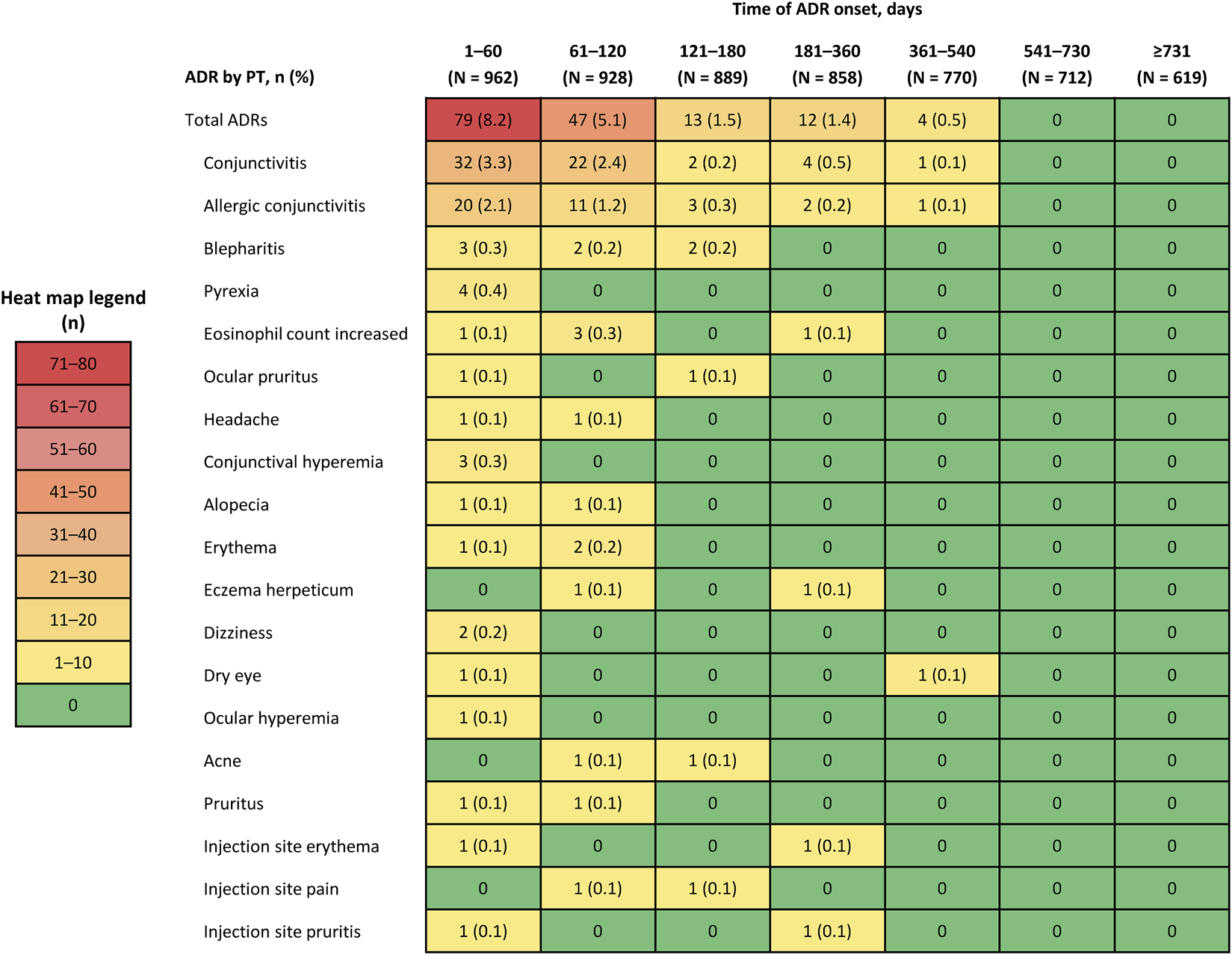
Incidence of adverse drug reactions (ADRs) that occurred in ≥2 patients by preferred term (PT) by time of onset during the observation period.
Six patients died during the course of the PMS. Causes of death were malignant neoplasm of the lung (76-year-old), cardiac death (81-year-old), aspiration pneumonia (81-year-old), renal failure (93-year-old), malignant neoplasm progression (75-year-old), and white blood cell count increased and myelodysplastic syndrome (65-year-old). For the first five patients, a causal relationship to this drug was ruled out by the investigators. For the sixth patient (with a white blood cell count increase and myelodysplastic syndrome), atypical lymphocytes were observed before dupilumab initiation and dupilumab was administered once; the event was considered causally related by the investigator.
For patients in whom dupilumab was readministered, the reasons for initial interruption, deferral, and discontinuation and the reasons for reinitiation were analyzed (Table 4). Overall, 724/962 patients (75.3%) had at least one event of either interruption, deferral, or discontinuation of dupilumab treatment during the observation period. The reasons of any such event included improvement of AD (125 patients; 17.3%), economic reasons (81 patients; 11.2%), occurrence of AEs (37 patients; 5.1%), inadequate response (20 patients; 2.8%), and other (460 patients; 63.5%; Table 4). Reasons for initial treatment reinitiation included AD flare (61 patients; 8.4%), economic reasons (40 patients; 5.5%), AE recovery (19 patients; 2.6%), and other (393 patients; 54.3%). In total, 513 patients (70.9%) reinitiated treatment at least once during the observation period; the median duration of either treatment interruption, deferral, or discontinuation was 95.0 days (IQR, 40.0–261.0). Of the 20 patients who once stopped dupilumab treatment due to inadequate clinical response, two patients (10.0%) reinitiated dupilumab. On the other hand, of the 125 patients who once stopped dupilumab due to improvement of AD, 88 patients (70.4%) reinitiated dupilumab, of which 45 patients (36.0%) reinitiated due to flare.
TABLE 4
| Reinitiation after first event of either treatment interruption, deferral, or discontinuation | Reasons for first events of either treatment interruption, deferral, or discontinuation | Overall, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Occurrence of AEs, n (%) | Improvement of primary disease, n (%) | Inadequate clinical response, n (%) | Economic reasons, n (%) | Other, n (%) | Unknowna, n (%) | |||
| Interruption, deferral, or discontinuation (n = 724) | 37/724 (5.1) | 125/724 (17.3) | 20/724 (2.8) | 81/724 (11.2) | 460/724 (63.5) | 1/724 (0.1) | 724 (100.0) | |
| Reinitiation of treatment | 21/37 (56.8) | 88/125 (70.4) | 2/20 (10.0) | 54/81 (66.7) | 347/460 (75.4) | 1/1 (100.0) | 513 (70.9) | |
| Reasons for reinitiation | Recovery from AE | 19 (51.4) | 0 | 0 | 0 | 0 | 0 | 19 (2.6) |
| Flare of primary disease | 0 | 45 (36.0) | 1 (5.0) | 7 (8.6) | 7 (1.5) | 1 (100.0) | 61 (8.4) | |
| Economic reasons | 0 | 0 | 0 | 40 (49.4) | 0 | 0 | 40 (5.5) | |
| Other reasons | 2 (5.4) | 43 (34.4) | 1 (5.0) | 7 (8.6) | 340 (73.9) | 0 | 393 (54.3) | |
Reasons for first events of either treatment interruption, deferral, or discontinuation; and reinitiation in the safety analysis set.
Patients who had >1 events for discontinuing or reinitiation are counted only once for their first event.
Blank responses are included in the column for unknown reasons for treatment interruption, deferral, or discontinuation.
AD, atopic dermatitis; AE, adverse event.
Among those who eventually discontinued dupilumab (n = 382), the median time to treatment discontinuation was 33.9 weeks (IQR, 15.6–64.0). Reasons for permanent treatment discontinuation included the improvement of AD (96 patients; 25.1%), economic reasons (54 patients; 14.1%), inadequate response (25 patients; 6.5%), occurrence of AEs (20 patients; 5.2%), and other (186 patients; 48.7%).
Effectiveness outcomes
Although the primary objective of this PMS was to investigate safety aspects, some effectiveness outcomes were collected as real-world data. The EASI score (mean ± SD) improved from 30.1 ± 13.2 at baseline to 10.7 ± 10.1 at 2 months and 4.0 ± 7.0 at 24 months (both P < 0.001 vs. baseline). Similarly, the proportion of patients who achieved EASI-75 increased from 44.1% at 2 months to 85.6% at 24 months (Figure 3A). Over the same period, EASI-50 increased from 75.5% to 95.1%, and EASI-90 increased from 16.9% to 63.5%.
FIGURE 3
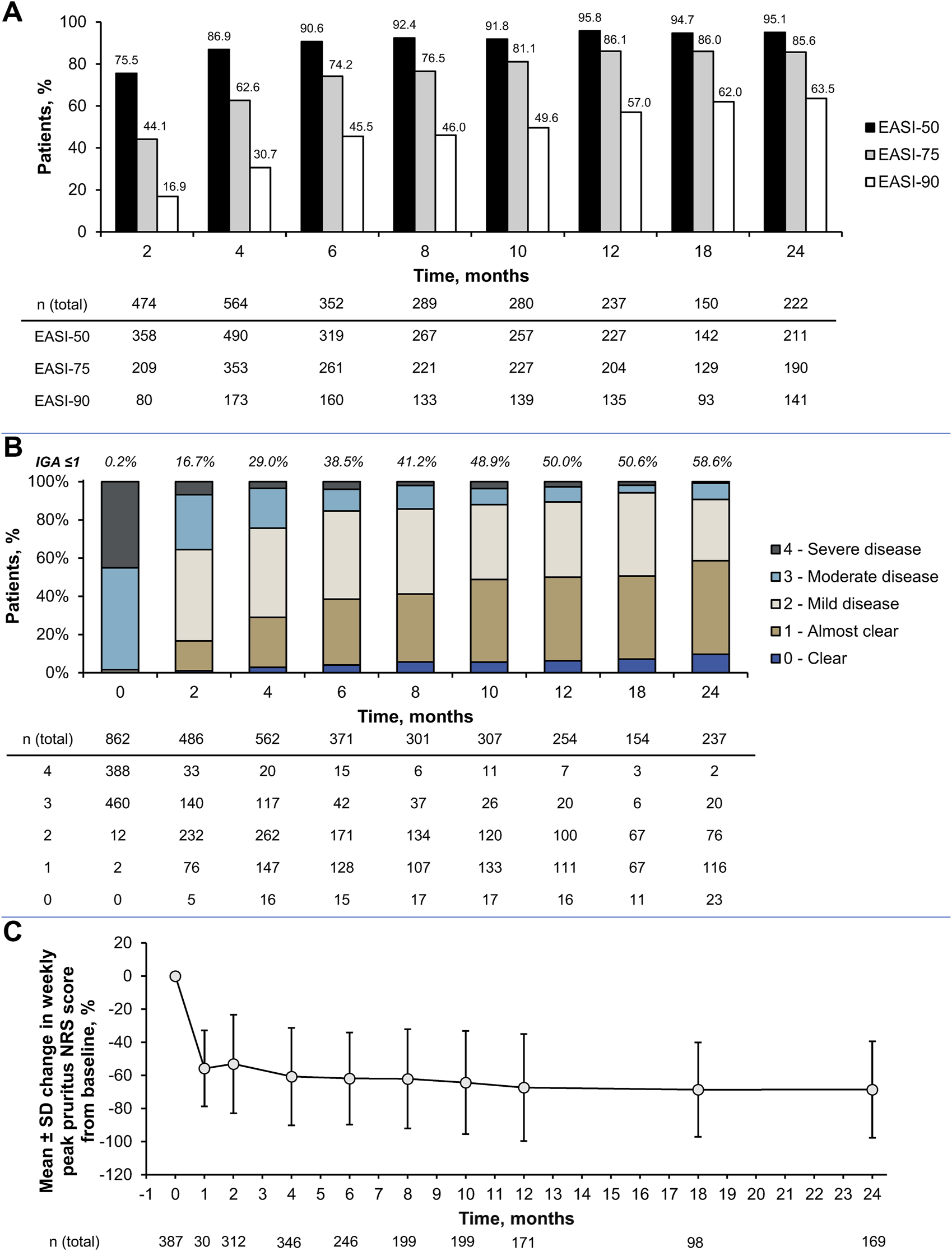
Effectiveness outcomes up to 2 years in the effective analysis set: (A) Improvements in Eczema Area and Severity Index (EASI), (B) changes in Investigator’s Global Assessment (IGA) score distribution and (C) percent change in weekly peak pruritus numerical rating scale (NRS) scores during the observation period. Data are based on the effectiveness analysis set (n = 892); error bars represent standard deviation. All scores were measured at baseline and at months 2, 4, 6, 8, 10, 12, 18, and 24. Despite the predetermined evaluation timepoint of 2 months on the case report forms (CRFs), some weekly peak pruritus NRS scores are shown for a small group (n = 30) at 1 month (when these were available on the CRFs) in (C). AD, atopic dermatitis; EASI-50, ≥50% improvement in Eczema Area and Severity Index; EASI-75, ≥75% improvement in Eczema Area and Severity Index; EASI-90, ≥90% improvement in Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; NRS, numerical rating scale; SD, standard deviation.
The mean IGA score also improved from 3.4 ± 0.5 at baseline to 2.2 ± 0.8 at 2 months and 1.4 ± 0.8 at 24 months (both P < 0.001 vs. baseline). The proportion of patients with an IGA score of 0 or 1 increased from 0.2% at baseline to 16.7% at 2 months and 58.6% at 24 months (Figure 3B). The weekly peak pruritus NRS score (mean ± SD) was 6.9 ± 2.1 at baseline, which improved to 3.1 ± 1.9 at 2 months and 1.8 ± 1.6 at 24 months (both P < 0.001 vs. baseline). This corresponded to percent changes (mean ± SD) of −53.1% ± 29.8% at 2 months and −68.5% ± 29.2% at 24 months (Figure 3C).
The proportion of patients who achieved an IGA score ≤1 and an IGA score reduction of ≥2 from baseline at any time during the observation period (effective proportion) was 47.4% (Supplementary Table S5). The effective proportion remained consistent across subgroups stratified by baseline age, age at AD onset, duration of AD, and the presence of allergic comorbidities. Female patients exhibited higher outcome achievement than male patients (55.4% vs. 44.0%).
Improvements in AD severity and symptoms with dupilumab treatment coincided with reductions in circulating biomarkers and patient-reported outcomes. The peripheral eosinophil count (mean ± SD) gradually decreased from 754.9 ± 765.9 cells/mm3 at baseline to 370.4 ± 335.5 cells/mm3 at 24 months (Supplementary Figure S3A). Apparent reductions in mean serum LDH, TARC, and total IgE from baseline were first achieved after 1 month of dupilumab treatment and continued to decrease over 24 months (P < 0.001 at all time points vs. baseline; Supplementary Figures S3B–D). Significant improvements in patient-reported DLQI, EQ-5D, and WPAI-AD scores were observed during the first 4 months after initiating dupilumab and were maintained through 1 year (Supplementary Table S6).
Discussion
The final results of this PMS confirmed the long-term safety and effectiveness of dupilumab in Japanese patients with AD not adequately controlled with conventional therapies such as topical therapy. Dupilumab was well tolerated and demonstrated an acceptable safety profile, and was associated with rapid and sustained improvements in AD signs, symptoms, biomarkers, and patient-reported outcomes up to 2 years. These data reconfirm the use of dupilumab for the treatment of moderate-to-severe AD in Japanese clinical practice.
One-year interim results from this PMS found that the incidence of ADRs was 16.4% and that serious ADRs occurred in 0.5% of patients [8]. In this final PMS analysis, the 2-year incidence of any ADR was 16.8% and of serious ADRs was 0.8%, suggesting that the risk of ADRs does not increase with ongoing dupilumab treatment. Notably, analyses that evaluated the incidence of ADRs over time found that most events occurred within 120 days of initiating dupilumab therapy.
The most common ADRs in this PMS were conjunctivitis (7.1%) and allergic conjunctivitis (4.2%), which has been a consistent finding among previous clinical trials (including subgroup analyses of Japanese participants [10]) and real-world studies of dupilumab in AD [11–17]. Because different studies have reported conjunctivitis events either as ADRs (such as the present study) or treatment-emergent AEs (such as in phase 3 clinical trials), we are not able to compare the rates from different studies. In the present study, the occurrences of all the ADRs were physician-reported and coded according to MedDRA. As such, conjunctivitis and allergic conjunctivitis ADRs are reported, as captured by investigators. Moreover, the incidence of AEIs (i.e., serious hypersensitivity reactions, serious infections, the exacerbation of symptoms associated with other allergic diseases, events associated with depression and suicidal behavior, and malignant tumors) was low or none in this PMS.
All eight cases of asthma AEs were reported in patients with a prior history of or comorbid asthma in this PMS, emphasizing the understanding of the existence of concomitant allergic conditions at dupilumab initiation. Seven cases were reported as mild, while one case as serious, which necessitated hospitalization. The duration of events in this subgroup was 58–572 days; however, the observation periods were typically longer than 700 days, except one permanently discontinued case after hospitalization due to exacerbation of asthma. Some cases may have occurred as a result of influenza infection, the common cold and/or COVID-19 vaccination. Rather than being assessed by pulmonologists, some events of exacerbation of asthma were collected according to symptoms corresponding to unscheduled visits, emergency visits, hospitalizations, and the administration of systemic steroids for 3 days or more. In addition, in this PMS, the details on the comorbid asthma control status of these patients were unclear.
This PMS also showed that the effectiveness of dupilumab observed in the 1-year interim analysis [8] was maintained over 2 years. More specifically, dupilumab treatment was associated with reductions in AD severity and symptoms, evidenced by significant improvements in EASI, IGA, and peak pruritus NRS scores over time. These improvements coincided with parallel reductions in serum LDH, TARC, and total IgE biomarker levels, which were first observed as early as after 1 month of dupilumab treatment and maintained over 24 months. Improvement in AD severity translated to increased productivity and health-related quality of life as shown by significant improvements in patient-reported DLQI, EQ-5D, and WPAI-AD scores over time.
Additional analyses found that the proportion of patients who achieved an IGA score ≤1 and an IGA score reduction of ≥2 at any time during the observation period (effective proportion) was comparable between subgroups stratified by several baseline factors (e.g., age, duration of AD, presence of allergic comorbidities), suggesting that dupilumab is effective across a wide range of people in a diverse AD population. It is interesting to note that, while a relatively large proportion of patients interrupted, deferred, or discontinued dupilumab treatment during the observation period, 71% of them reinitiated treatment. Regarding the reason for interruption, deferral, and discontinuation in Table 4, of the 460 events classified as “other,” 347 events resulted in treatment resumption, indicating continuous visits to the same medical institution, rather than being due to unavoidable physical circumstances such as relocation. This suggests that many interruptions with “other” reason were likely temporary interruptions, deferrals, or discontinuations due to patient circumstances (not showing up or patients request). On the other hand, analysis of known interruption, deferral, and discontinuation reasons showed that (1) low resumption rate when the reason was insufficient clinical efficacy (2/20, 10.0%), and (2) high resumption rate when the reason was symptom improvement (88/125, 70.4%). Based on these resumption patterns of physician-documented interruptions, deferrals, or discontinuations, it may be inferred that many of the events classified as “other” that resulted in treatment resumption were likely due to patient-determined symptom improvement. In addition, the median duration of active dupilumab treatment during the observation period was 62.3 weeks, indicating that dupilumab could have remained a viable option for most patients, even in case of discontinuation, deferral, or interruption. Given that AD is a long-lasting chronic disease that requires careful disease control, the establishment of a long-term safety and effectiveness profile is critical.
A key strength of this PMS was that it demonstrated the real-world safety and effectiveness of dupilumab in the largest prospective cohort of people with moderate-to-severe AD reported in Japan [8]. As mentioned previously, real-world studies allow treatments to be evaluated in populations and settings that reflect current clinical practice.
Nevertheless, this PMS has its limitations. The study may have been limited by its observational, non-comparative design, therefore, causation could not be ascertained. Due to the inclusion of Japanese patients only, the generalizability of the findings is limited to the Japanese population under a given health insurance system. The results may have been affected by factors unrelated to dupilumab (e.g., the use of concomitant treatments for AD during the observation period and since follow-up visits were not compulsory, patients may have dropped out for reasons unrelated to dupilumab). Biases may have been introduced because this study was funded by a pharmaceutical company that markets dupilumab and sites were paid to conduct surveys, implying a possible conflict of interest. There were also no specific details on the large percentage of patients whose reasons for treatment interruption, deferral, or discontinuation were classified as “other,” which could affect the interpretation of the results.
In conclusion, updated results from this PMS have confirmed that the safety and effectiveness of dupilumab therapy for AD are maintained over 2 years in Japanese clinical practice. These results suggest that dupilumab represents a safe and effective strategy to improve and sustain AD-related signs, symptoms and quality of life for people with moderate-to-severe AD in Japan.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committees/institutional review boards of the individual facilities and the Japan Conference of Clinical Research. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HS, KS, and KA contributed to the study design. HS enrolled patients. MU and KS contributed to the data analysis, and all authors contributed to interpretation of the data, critically revised the manuscript for intellectual content, and approved the final version of the manuscript for publication. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study (OBS15953) and the open access fee were funded by Sanofi K.K. The study sponsor participated in the study design, the collection, analysis and interpretation of the data, and the writing of the report. This editorial support was funded by Sanofi K.K.
Acknowledgments
The authors would like to thank Masayuki Senda of Sanofi K.K. for data collection, and Karina Hamilton-Peel, PhD, CMPP, and Iona MacDonald of inScience Communications, Springer Healthcare, who assisted with preparation of the draft manuscript.
Conflict of interest
All the authors have filled out an International Committee of Medical Journal Editors (ICMJE) conflict of interest form. HS was an external advisor and received honoraria for lectures from AbbVie, Kyorin Pharmaceutical, Kyowa Kirin, Maruho, Mitsubishi Tanabe Pharma, Sanofi, and Taiho Pharma; and has received research grants from Eisai, Tokiwa Pharmaceutical, and Torii Pharmaceutical. MU, KS, MM, and KA are employees and may be shareholders of Sanofi K.K.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jcia.2025.14794/full#supplementary-material
Abbreviations
AD, atopic dermatitis; ADR, adverse drug reaction; AE, adverse event; AEI, adverse event of interest; CCL, C-C motif chemokine ligand; CI, confidence interval; CRF, case report form; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; EQ, EuroQol; HLGT, high level group term; Ig, immunoglobulin; IGA, Investigator’s Global Assessment; IL, interleukin; IQR, interquartile range; J-RMP, Japanese Risk Management Plan; LDH, lactate dehydrogenase; MedDRA, Medical Dictionary for Regulatory Activities; NRS, numerical rating scale; PMS, post-marketing surveillance; PT, preferred term; SD, standard deviation; SMQ, standardized MedDRA query; SOC, system organ class; TARC, thymus and activation-regulated chemokine; WPAI-AD, Work Productivity and Activity Impairment Questionnaire.
References
1.
LanganSMIrvineADWeidingerS. Atopic dermatitis. Lancet (2020) 396(10247):345–60. 10.1016/s0140-6736(20)31286-1
2.
SaekiHOhyaYFurutaJArakawaHIchiyamaSKatsunumaTet alExecutive summary: Japanese guidelines for atopic dermatitis (ADGL) 2021. Allergol Int (2022) 71(4):448–58. 10.1016/j.alit.2022.06.009
3.
DarlenskiRKazandjievaJHristakievaEFluhrJW. Atopic dermatitis as a systemic disease. Clin Dermatol (2014) 32(3):409–13. 10.1016/j.clindermatol.2013.11.007
4.
KabashimaK. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci (2013) 70(1):3–11. 10.1016/j.jdermsci.2013.02.001
5.
KamataMTadaY. Optimal use of JAK Inhibitors and biologics for atopic dermatitis on the basis of the current evidence. JID Innov (2023) 3(3):100195. 10.1016/j.xjidi.2023.100195
6.
Le Floc'hAAllinneJNagashimaKScottGBirchardDAsratSet alDual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy (2020) 75(5):1188–204. 10.1111/all.14151
7.
HamiltonJDHarelSSwansonBNBrianWChenZRiceMSet alDupilumab suppresses type 2 inflammatory biomarkers across multiple atopic, allergic diseases. Clin Exp Allergy (2021) 51(7):915–31. 10.1111/cea.13954
8.
SaekiHFujitaHSuzukiKArimaK. Safety and effectiveness of dupilumab in the real‐world treatment of atopic dermatitis in Japan: 1‐year interim analysis from a post‐marketing surveillance. J Cutan Immunol Allergy (2023) 6(3):78–87. 10.1002/cia2.12303
9.
Pharmaceutical and Medical Devices Agency. Pharmaceutical risk management plan for Dupixent subcutaneous injection 300mg syringe, Dupixent subcutaneous injection 300mg pen, and Dupixent subcutaneous injection 200mg syringe (2025). Available online at: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/4490405 (Accessed April 14, 2025).
10.
KatohNKataokaYSaekiHHideMKabashimaKEtohTet alEfficacy and safety of dupilumab in Japanese adults with moderate-to-severe atopic dermatitis: a subanalysis of three clinical trials. Br J Dermatol (2020) 183(1):39–51. 10.1111/bjd.18565
11.
HallingASLoftNSilverbergJIGuttman-YasskyEThyssenJP. Real-world evidence of dupilumab efficacy and risk of adverse events: a systematic review and meta-analysis. J Am Acad Dermatol (2021) 84(1):139–47. 10.1016/j.jaad.2020.08.051
12.
VittrupIKroghNSLarsenHHPElberlingJSkovLIblerKSet alA nationwide 104 weeks real-world study of dupilumab in adults with atopic dermatitis: ineffectiveness in head-and-neck dermatitis. J Eur Acad Dermatol Venereol (2023) 37(5):1046–55. 10.1111/jdv.18849
13.
AlraddadiRAlsamadaniAHKalantanMAAljefriYEMaaddawiHAKadasaANet alIncidence of conjunctivitis adverse event in patients treated with biologics for atopic dermatitis: a systematic review and meta-analysis. JAAD Int (2023) 13:46–7. 10.1016/j.jdin.2023.05.014
14.
FachlerTShreberk-HassidimRMolho-PessachV. Dupilumab-induced ocular surface disease: a systematic review. J Am Acad Dermatol (2022) 86(2):486–7. 10.1016/j.jaad.2021.09.029
15.
NeaguNDianzaniCAvalloneGDell'AquilaCMorariuSHZalaudekIet alDupilumab ocular side effects in patients with atopic dermatitis: a systematic review. J Eur Acad Dermatol Venereol (2022) 36(6):820–35. 10.1111/jdv.17981
16.
AugustinMBauerAErtnerKvon KiedrowskiRSchenckFRamaker-BrunkeJet alDupilumab demonstrates rapid onset of action in improving signs, symptoms and quality of life in adults with atopic dermatitis. Dermatol Ther (Heidelb) (2023) 13(3):803–16. 10.1007/s13555-023-00894-3
17.
SimpsonELLockshinBLeeLWChenZDaoudMKorotzerA. Real-world effectiveness of dupilumab in adult and adolescent patients with atopic dermatitis: 2-year interim data from the PROSE registry. Dermatol Ther (Heidelb) (2024) 14(1):261–70. 10.1007/s13555-023-01061-4
Summary
Keywords
atopic dermatitis, dupilumab, post-marketing surveillance, quality of life, real-world evidence
Citation
Saeki H, Usami M, Suzuki K, Mochizuki M and Arima K (2025) Real-world safety and effectiveness of dupilumab for the treatment of atopic dermatitis: two-year post-marketing surveillance in Japan. J. Cutan. Immunol. Allergy 8:14794. doi: 10.3389/jcia.2025.14794
Received
21 April 2025
Accepted
05 June 2025
Published
09 July 2025
Volume
8 - 2025
Updates
Copyright
© 2025 Saeki, Usami, Suzuki, Mochizuki and Arima.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazuhiko Arima, kazuhiko.arima@sanofi.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.