Introduction
Obesity significantly increases the risk of complications following abdominal wall reconstruction (AWR), including surgical site infections (SSIs), wound dehiscence, and hernia recurrence rates of up to 35%–40% [1–3]. A BMI threshold of BMI ≥35 kg/m2 has typically been identified as a threshold at which these risks rise substantially; however, prior data has shown that there is an increase starting at lower BMIs [4, 5]. With global obesity rates continuing to rise, hernia surgeons are increasingly tasked with managing high-risk patients whose comorbidities and functional limitations require careful optimization prior to surgery when possible [6]. AWR is a benign procedure performed to restore function and improve quality of life rather. This distinction underscores the importance of maximizing perioperative outcomes, as the central goal is to achieve durable repair and enhance functionality. Despite this, there is currently no standardized, stepwise approach to prehabilitation that incorporates endoscopic bariatric therapies. This gap in clinical practice highlights an opportunity to integrate endoscopic weight-loss strategies, specifically endoscopic sleeve gastroplasty (ESG), into the preoperative pathway for patients undergoing AWR. This manuscript explores the rationale, benefits, limitations, and practical considerations of ESG as a potential prehabilitation strategy to improve outcomes in this high-risk population.
Rationale for Weight Loss Prior to Abdominal Wall Reconstruction
Weight loss prior to AWR improves both technical and clinical outcomes [7]. Reducing visceral and subcutaneous adiposity can facilitate fascial approximation, reduce tension at the repair site, and lower the risk of postoperative complications [8]. Enhancing a patient’s functional capacity before surgery can also contribute to optimal outcomes by improving cardiopulmonary function, glycemic control, and overall patient resilience, factors that collectively reduce surgical risk and improve the overall health of the patient [5, 9]. In our practices at University of Texas Health Austin and Endeavor Health, implementation of a ketogenic diet supplemented by daily aerobic exercise has been effective in optimizing patients for surgery and resulted in sustained improvements in health following surgery [10]. While we do not have a specific cut point for body mass index (BMI) prior to open AWR, in general, we encourage weight loss in all overweight and obese patients as each unit of BMI is associated with a significant reduction in wound complications [3]. In patients with large hernias, weight loss has the important impact of facilitating fascial closure by reducing hernia and intra-abdominal volume, and it also reduces tension on the closed wound. Previous studies demonstrate that lack of fascial closure increases recurrence rates by 7-fold and that increasing obesity directly correlates with elevated intra-abdominal pressure [11, 12]. Traditionally, these lifestyle modifications (e.g., ketogenic diet and daily aerobic exercise) have been the cornerstone of perioperative weight management, whereas surgical or endoscopic weight loss interventions were typically reserved for patients with more severe obesity or those without urgent indications for hernia repair. The purpose of this opinion piece is to advocate for the inclusion of ESG as a complementary strategy within the existing perioperative optimization paradigm. In particular, this may be a valuable emerging technology to achieve a longer-term effect in comparison to lifestyle modifications alone.
Primary Endobariatric Therapies and Clinical Benefits
Two principal endobariatric options for weight loss include intragastric balloon (IGB) therapy and endoscopic sleeve gastroplasty (ESG). IGB involves the endoscopic placement of a saline-filled or air-filled balloon in the stomach, which induces early satiety and reduces caloric intake. By far, the most commonly used ballon in the United States, the OrberaTM (Boston Scientific, El Coyol, Costa Rica) [13]. IGB is Food and Drug Administration (FDA) approved for a maximum duration of 6 months. During this timeframe, it can induce an excess weight loss of 30%–40% [14]. However, this weight loss is typically not durable, and weight often recurs once the balloon is removed. Given these limitations, ESG is the preferred endoscopic intervention for patients requiring more sustained weight loss and metabolic improvement. That said, for carefully selected patients who required rapid, short term weight reduction without permanent anatomical changes, IGB still remains a viable option.
Although longer-term follow-up is needed, ESG may be considered as an endobariatric approach in the AWR setting. ESG involves transoral placement of full thickness sutures to reduce the stomach’s size by 70%–80%, mimicking the restrictive anatomy of a surgical sleeve gastrectomy that limits food intake and induces changes in gastrointestinal hormones involved in satiety without the need for resection [15]. Importantly, this technique avoids entering the peritoneal cavity, which is particularly advantageous in patients with prior abdominal surgeries or mesh implantation. ESG produces most of the weight loss within 6 months, which is beneficial for a patient who is being optimized for a hernia repair. In the multi-center ESG Randomized Interventional Trial (MERIT-Trial), investigators enrolled 209 participants with class 1 or class 2 obesity (BMI 30–40 kg/m2) to assess the effects of ESG combined with lifestyle modifications [15]. After 52 weeks, the primary endpoint of 25% excess weight loss (EWL) was met in 77% of patients in the ESG group compared to only 12% in the control group (lifestyle modifications). Put differently, the mean percentage of total body weight loss at 52 weeks was 13.6% for the ESG group and 0.8% for the control group. Impressively, 68% of patients undergoing ESG maintained their weight loss at 2 years. ESG was well tolerated, with only 2% of participants experiencing serious adverse events (e.g., severe nausea, vomiting, and abdominal pain), none of which were life-threatening. The overall rate of major complications from sleeve gastrectomy is approximately 2%–7% (e.g., staple line leak, bleeding stricture or stenosis, and gastroesophageal reflux). Recent systematic review and meta-analysis yielded similar results with a 16.2% TWL at 12 months [16]. These results reinforce ESG as an effective and safe intervention for achieving sustainable weight loss in patients with obesity when compared to lifestyle modifications.
Beyond weight loss, ESG offers clinically significant benefits in metabolic health. In the MERIT trial, over 90% of the patients experienced clinical improvement in their diabetes, with no deterioration. A separate systematic review by Nunes et al. demonstrated that ESG is associated with significant improvement in hepatic steatosis, liver fibrosis, anthropometric measurements, and a reduction in HbA1c over 12 months of follow-up [17]. Such systemic improvements enhance surgical candidacy and long-term health outcomes, making ESG a compelling tool in multidisciplinary, prehabilitation protocols.
Glucagon-Like-Peptide-1 (GLP-1) Agonist
The emerging data on GLP-1s in the context of AWR are also playing an increasingly prominent role in AWR prehabilitation. Their key benefit is immediacy; patients can initiate therapy promptly and begin losing weight without procedural clearance delays. The use of GLP-1s has demonstrated accelerated weight loss compared to lifestyle modifications alone and compress the timeframe to surgery [18, 19]. In a recently published prospective, single-institution hernia database analysis, patients who were optimized through diet and exercise alone took an average of 10 months to get to surgery while patients on GLP-1s have reached optimization targets at just over 6 months [10, 18]. Although there is no level 1 data directly comparing the weight loss effects of ESG and GLP-1s, GLP-1s may be particularly useful for patients who are not candidates for endoscopic procedures and require rapid preoperative optimization. This recommendation reflects considerations beyond weight loss alone, including procedural access, patient preferences, and the relative invasiveness of ESG compared with pharmacologic therapy.
GLP-1s do, however, have limitations. Medication tolerance is a significant concern; over one-third of patients experience significant gastrointestinal side effects, such as nausea and vomiting [20]. It should be noted that similar gastrointestinal symptoms (e.g., nausea and vomiting) have been reported following ESG; however, symptoms are typically limited to the immediate post-procedural period, as demonstrated in the MERIT trial and confirmed in systematic review [21]. Additional contraindications to GLP-1 therapy include a history of pancreatitis, and, in some instances, chronic kidney disease [22]. Furthermore, the need for ongoing administration of the medication to sustain benefits presents a practical and financial challenge. When not covered by insurance, the out-of-pocket cost for GLP-1 medications in the U.S. frequently exceeds $1,000 per month, making long-term use financially burdensome [23]. However, the use of GLP-1s may serve as a complimentary treatment to ESG and other lifestyle modifications.
Proposed Algorithm
Figure 1 offers a practical algorithm for optimizing obese AWR patients prior to elective surgery. For all overweight patients and those with comorbidities (e.g., diabetes, NAFLD), initiate lifestyle modifications (ketogenic diet, aerobic exercise) and assess surgical urgency. If weight loss plateaus or urgency is low, consider ESG for BMI above 30 kg/m2 and ESG or bariatric surgery for patients with a BMI above 35 kg/m2 and with comorbidities. ESG would be the preferred treatment option in a patient with multiple prior abdominal surgeries. IGB would generally not be recommended as a first line procedural intervention given its lack of durability. GLP-1 agonists may be beneficial for more urgent cases or ESG-ineligible patients (e.g., prior gastric surgery). Concurrently, surgeons can address smoking cessation and glycemic control. For patients who have had recurrent episodes of incarceration, they may not be able to be optimized and require surgery prior to weight loss interventions. This algorithm requires validation through multicenter trials to optimize patient selection and outcomes. American and European Hernia Society-led initiatives should standardize endoscopic training to facilitate adoption as part of the algorithm to optimize obese patients. As of now, the defined optimization standards for minimally invasive abdominal wall reconstruction are less clear and may affect this algorithm.
FIGURE 1
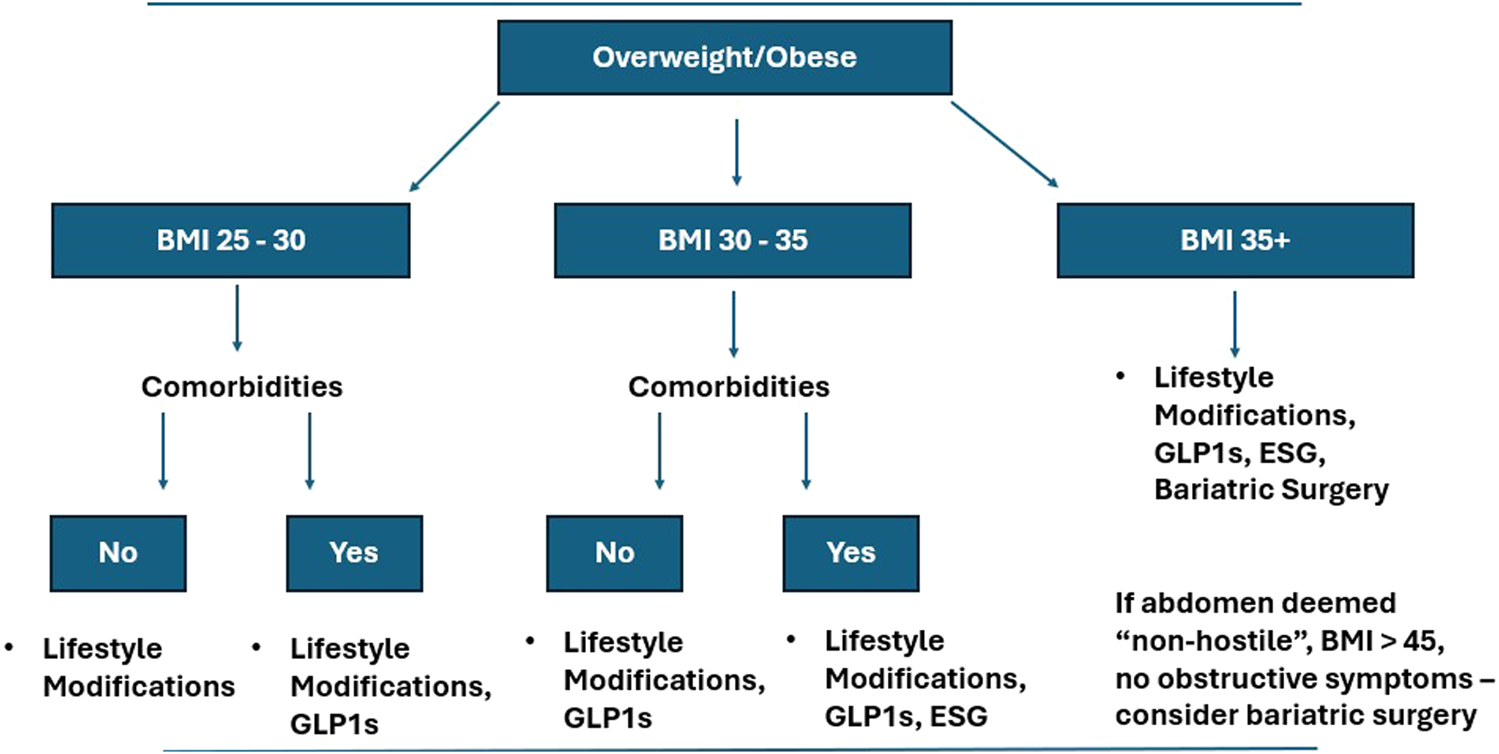
Algorithm for addressing obesity in abdominal wall reconstruction patients. The figure demonstrates a proposed algorithm for optimizing obese patients prior to abdominal wall reconstruction. The algorithm accounts for body mass index, comorbidities, and prior surgical history.
Discussion
ESG offers several unique advantages over traditional bariatric surgery in the setting of AWR. Unlike LSG or Roux-en-Y gastric bypass (LRYGB), ESG is incisionless and avoids intra-abdominal entry, which is important in patients with a hostile abdomen, prior mesh placement, etc. It is also associated with a shorter recovery time and reduced hospital stay, with most patients being discharged the same day and returning to normal activities shortly thereafter [15]. While LSG may provide greater weight loss than ESG in the long term, ESG demonstrates comparable improvement in comorbidities and superior procedural safety in the prehabilitation context [24]. LRYGB is less frequently performed in this setting because a hernia-related issue may lead to significant problems with intra-abdominal surgery, bariatric-related anatomy, and the possibility of future RYGB related surgery. ESG is particularly suited for patients with elevated BMI and diminished abdominal wall compliance, in whom even modest weight reduction can improve closure dynamics and reduce operative tension [25]. Additionally, ESG facilitates meaningful improvement in metabolic parameters, particularly diabetes and hepatic steatosis, thereby further reducing perioperative risk. As a same-day procedure with rapid recovery, ESG minimizes delays in care and can be initiated during the preoperative workup without major disruptions to the hernia surgical timeline. As endoscopic approaches to obesity management, particularly ESG, become mainstream, they have the potential to become an important part in the prehabilitation of patients undergoing complex hernia repair. While weight loss is only one component of comprehensive optimization (e.g., physical therapy, smoking cessation, glycemic control), surgeons who understand and utilize endoscopic options will be better positioned to offer individualized, effective care. Expanding the number of surgeons trained in endoscopic techniques has the ability to empower them to deliver integrated, in-house solutions that improve both access and outcomes. The successful integration of endobariatrics into clinical practice will ultimately depend on addressing issues such as insurance coverage, specialized training, and thoughtful patient selection. At present, ESG is not always covered by major commercialized health plans, but this is becoming less common as there is mounting evidence regarding its efficacy [26]. For these patients, bariatric surgery remains the gold standard procedural means for prehabilitation. The goal of this algorithm is to describe the role of endoscopic therapy as a potential option rather than assert its superiority to bariatric surgery, which is not the case.
Despite the promise that ESG may offer in the context of preoptimization, access remains a barrier. Many insurers do not deem ESG medically necessary, limiting access to patents who cannot pay out-of-pocket [27]. Although the defined and standardized code for reimbursement for ESG is forthcoming, its adoption into routine practice may be slowed by a lack of formally trained endoscopists. ESG requires specialized and advanced endoscopic skills not typically included in general surgery or gastroenterology training and often require specialized gastrointestinal or minimally invasive surgery fellowships or society-sponsored courses. These challenges may inevitably contribute to a slower uptake of ESG in hernia prehabilitation, despite its clear potential to improve patient outcomes. Surgeons who do offer ESG will be posed to uniquely take care of patients in the preoperative setting. As the aim of elective AWR is to improve patient quality of life, providing patients with the chance to have the optimal outcome and improve their overall health is paramount.
Statements
Author contributions
VW, SK, BH, and SA and student doctor GL all contributed to the conceptualization, drafting, and editing of the article. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
BH receives education grant funding and honoraria from WL. Gore. SA receives education grant funding from Boston Scientific. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Tastaldi L Krpata DM Prabhu AS Petro CC Rosenblatt S Haskins IN et al The Effect of Increasing Body Mass Index on Wound Complications in Open Ventral Hernia Repair with Mesh. The Am J Surg (2019) 218(3):560–6. 10.1016/j.amjsurg.2019.01.022
2.
Heniford BT Ross SW Wormer BA Walters AL Lincourt AE Colavita PD et al Preperitoneal Ventral Hernia Repair: A Decade Long Prospective Observational Study with Analysis of 1023 Patient Outcomes. Ann Surg (2020) 271(2):364–74. 10.1097/sla.0000000000002966
3.
Giordano SA Garvey PB Baumann DP Liu J Butler CE . The Impact of Body Mass Index on Abdominal Wall Reconstruction Outcomes: A Comparative Study. Plast & Reconstr Surg (2018) 139(5):1234–44. 10.1097/prs.0000000000003264
4.
Farhan SA Farhan SH Janis JE . Does BMI Impact Outcomes in Patients Undergoing Open Abdominal Wall Reconstruction? A Systematic Review and Meta‐Analysis. World J Surg (2025) 49(7):1777–86. 10.1002/wjs.12649
5.
Augenstein VA Colavita PD Wormer BA Walters AL Bradley JF Lincourt AE et al Cedar: Carolinas Equation for Determining Associated Risks. J Am Coll Surg (2015) 221(4):S65–6. 10.1016/j.jamcollsurg.2015.07.145
6.
Ng M Gakidou E Lo J Abate YH Abbafati C Abbas N . Global, Regional, and National Prevalence of Adult Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. The Lancet (2025) 405(10481):813–38. 10.1016/s0140-6736(25)00355-1
7.
Rodrigues-Gonçalves V Verdaguer-Tremolosa M Martínez-López P Nieto C Khan S López-Cano M . Obesity-Focused Prehabilitation Strategies in Ventral Hernia: Cohort Study. Hernia (2025) 29:202. 10.1007/s10029-025-03392-x
8.
Li J Wu L Shao X . Impact of Body Fat Location and Volume on Incisional Hernia Development and Its Outcomes Following Repair. ANZ J Surg (2024) 94(5):804–10. 10.1111/ans.18873
9.
Ayuso SA Elhage SA Fischer JP Heniford BT . The Role of Prehabilitation in Abdominal Wall Reconstruction: It Is More than “Watch and Wait”. Ann Surg Open (2024) 5(2):e449. 10.1097/as9.0000000000000449
10.
Holland AM Lorenz WR Ayuso SA Katzen MM Kundu S Rosas DA et al Limited or Lasting: Is Preoperative Weight Loss as Part of Prehabilitation Maintained After Open Ventral Hernia Repair? J Am Coll Surgeons (2025) 241(2):171–9. 10.1097/xcs.0000000000001348
11.
Pauli EM Wang J Petro CC Juza RM Novitsky YW Rosen MJ . Posterior Component Separation with Transversus Abdominis Release Successfully Addresses Recurrent Ventral Hernias Following Anterior Component Separation. Hernia (2014) 19(2):285–91. 10.1007/s10029-014-1331-8
12.
Cobb WS Burns JM Kercher KW Matthews BD James Norton H Todd HB . Normal Intraabdominal Pressure in Healthy Adults. J Surg Res (2005) 129(2):231–5. 10.1016/j.jss.2005.06.015
13.
Dave N Dawod E Simmons OL . Endobariatrics: A Still Underutilized Weight Loss Tool. Curr Treat Options Gastro (2023) 21(2):172–84. 10.1007/s11938-023-00420-6
14.
Kotzampassi K Grosomanidis V Papakostas P Penna S Eleftheriadis E . 500 Intragastric Balloons: What Happens 5 Years Thereafter?OBES SURG (2012) 22(6):896–903. 10.1007/s11695-012-0607-2
15.
Abu Dayyeh BK Bazerbachi F Vargas EJ Sharaiha RZ Thompson CC Thaemert BC et al Endoscopic Sleeve Gastroplasty for Treatment of Class 1 and 2 Obesity (MERIT): A Prospective, Multicentre, Randomised Trial. The Lancet (2022) 400(10350):441–51. 10.1016/S0140-6736(22)01280-6
16.
Fehervari M Fadel MG Alghazawi LOK Das B Rodriguez-Luna MR Perretta S et al Medium-Term Weight Loss and Remission of Comorbidities Following Endoscopic Sleeve Gastroplasty: A Systematic Review and Meta-Analysis. OBES SURG (2023) 33(11):3527–38. 10.1007/s11695-023-06778-x
17.
Nunes BCM De Moura DTH Kum AST De Oliveira GHP Hirsch BS Ribeiro IB et al Impact of Endoscopic Sleeve Gastroplasty in Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. OBES SURG (2023) 33(9):2917–26. 10.1007/s11695-023-06747-4
18.
Spurzem GJ Broderick RC Ruiz-Cota P Hollandsworth HM Sandler BJ Horgan S et al GLP-1 Receptor Agonists Are a Transformative Prehabilitation Tool for Weight Loss in Obese Patients Undergoing Elective Hernia Repair. Surg Endosc (2024) 39(1):440–7. 10.1007/s00464-024-11308-6
19.
Tran D Farias DA Tanner M Marroquin M Jefferies RS Ogola GO et al Impact of Glucagon-Like Peptide-1 Agonists in Optimizing Abdominal Wall Reconstruction Patients. Hernia (2024) 29(1):19. 10.1007/s10029-024-03214-6
20.
Trujillo JM Nuffer W Smith BA . GLP-1 Receptor Agonists: An Updated Review of Head-to-Head Clinical Studies. Ther Adv Endocrinol (2021) 12:2042018821997320. 10.1177/2042018821997320
21.
Stier CK Téoule P Dayyeh BKA . Endoscopic Sleeve Gastroplasty (ESG): Indications and Results—A Systematic Review. Updates Surg (2025). 10.1007/s13304-025-02097-1
22.
Long B Pelletier J Koyfman A Bridwell RE . GLP-1 Agonists: A Review for Emergency Clinicians. The Am J Emerg Med (2024) 78:89–94. 10.1016/j.ajem.2024.01.010
23.
Docimo S Shah J Warren G Ganam S Sujka J Ducoin C . A Cost Comparison of GLP-1 Receptor Agonists and Bariatric Surgery: What Is the Break Even Point?Surg Endosc (2024) 38(11):6560–5. 10.1007/s00464-024-11191-1
24.
Joseph S Vandruff VN Amundson JR Che S Zimmerman C Ishii S et al Comparable Improvement and Resolution of Obesity-Related Comorbidities in Endoscopic Sleeve Gastroplasty vs Laparoscopic Sleeve Gastrectomy: Single-Center Study. Surg Endosc (2024) 38(10):5914–21. 10.1007/s00464-024-11194-y
25.
Baratte C Sebbag H Arnalsteen L Auguste T Blanchet MC Reche F et al Position Statement and Guidelines About Endoscopic Sleeve Gastroplasty (ESG) Also Known as Endo-Sleeve. J Visc Surg (2025) 162:71–8. 10.1016/j.jviscsurg.2024.12.003
26.
Sharaiha RZ Wilson EB Zundel N Ujiki MB Dayyeh BKA . Randomized Controlled Trial Based US Commercial Payor Cost-Effectiveness Analysis of Endoscopic Sleeve Gastroplasty Versus Lifestyle Modification Alone for Adults with Class I/II Obesity. OBES SURG (2024) 34(9):3275–84. 10.1007/s11695-024-07324-z
27.
Vannucci M Riva P Vix M Mutter D Keller DS Perretta S . Endoscopic Sleeve Gastroplasty Versus Lifestyle Modifications for Class II Obesity Patients: A French Cost-Effectiveness Analysis. Surg Endosc (2025) 39(2):1333–40. 10.1007/s00464-024-11487-2
Summary
Keywords
ventral hernia, abdominal wall reconstruction, prehabilitation, obesity, endoscopic sleeve gastroplasty
Citation
Walker VL, Kerr SW, LaFleur GN, Heniford BT and Ayuso SA (2025) The Endoscopic Optimization for Obesity in Patients Undergoing Abdominal Wall Reconstruction. J. Abdom. Wall Surg. 4:15383. doi: 10.3389/jaws.2025.15383
Received
04 August 2025
Accepted
15 October 2025
Published
24 October 2025
Volume
4 - 2025
Updates
Copyright
© 2025 Walker, Kerr, LaFleur, Heniford and Ayuso.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sullivan A. Ayuso, sullivan.ayuso@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.