Abstract
Background:
The Da Vinci Single Port (SP) robotic platform has recently been approved for general surgery in the European Union. However, its application in abdominal wall hernia repair remains largely unexplored. This study focuses on the development of a novel suprapubic single-port extraperitoneal approach (SP2 eTEP) for ventral hernia surgery.
Method:
Following the IDEAL framework for introducing new surgical procedures, this study details the preclinical exploration and technical development of SP2eTEP using the Da Vinci SP system. The research included procedural development on human cadavers and skills training using a porcine model.
Results:
Instrument reach and maneuverability were first evaluated using a silicone abdominal wall model. The model showed minimal reach loss between the 27 mm-SP metal cannula (29 cm straight, 27 cm articulated) and the collapsed SP small access port (28 cm straight, 26 cm articulated). Cadaveric sessions confirmed that the SP Access Port, placed suprapubically, allowed successful dissection in the preperitoneal, the retrorectus, and the subcutaneous planes. Additionally, bilateral component separation by transversus abdominis release was achieved using a bottom-up approach. The porcine model for inguinal hernia repair proved to be a suitable adjunct to simulator-based training for developing the necessary skills for subsequent clinical application.
Conclusion:
This preclinical (pre-IDEAL stage) exploration and procedural development demonstrate the feasibility of SP2 eTEP for ventral hernia repair with the Da Vinci SP platform. The findings support progression to clinical evaluation of this novel robotic approach for abdominal wall hernia repair.
Introduction
Ventral hernia repair is a high-volume surgical procedure, increasingly performed using minimally invasive surgery (MIS) due to its association with reduced wound complications, faster recovery, and shorter hospital stays compared to open repair [1, 2]. Historically, laparoscopic intraperitoneal onlay mesh (IPOM) repair was the dominant MIS technique [3], but concerns about mesh-related complications—adhesions, bowel injury, and chronic pain from transfascial fixation—have led to a paradigm shift toward extraperitoneal mesh placement [4–7]. Recent evidence and international guidelines now favor sublay repairs with mesh placed in preperitoneal or retromuscular planes, which are associated with fewer complications and potentially improved long-term outcomes [1, 5, 8]. However, achieving these anatomical planes using conventional laparoscopy is technically demanding. Limitations in instrument articulation and visual angles, particularly for anterior wall dissection, have restricted adoption of extraperitoneal techniques such as transabdominal preperitoneal repair (TAPP), transabdominal retromuscular mesh repair (TARM/TARUP), extended totally extraperitoneal repair (eTEP), preperitoneal extended totally extraperitoneal technique (PeTEP), transversus abdominis release repair (TAR) and subcutaneous endoscopic onlay repair (SCOLA/ENDOR) [8–14]. The introduction of robotic platforms has significantly expanded the feasibility of complex MIS hernia repair [15, 16]. Robotic-assisted ventral hernia repair enables high-precision dissection, intracorporeal suturing, and advanced reconstruction techniques such as TAR via minimally invasive approaches [15, 17, 18]. These advancements have driven broader uptake of robotic extraperitoneal techniques, including robotic (P)eTEP and TAR, particularly in patients with midline or incisional defects [19–22]. Most robotic approaches to the abdominal wall currently rely on multi-port systems with lateral approaches. Recently, suprapubic approaches with cranial dissection have shown promise for accessing retrorectus and preperitoneal planes, offering ergonomic advantages and improved access to the lower midline [20, 23]. The Da Vinci Single Port (SP) platform—approved for general surgery in the European Union—introduces a compact, single-cannula system with flexible, wristed instruments that may further reduce tissue trauma and enable novel approaches to the anterior abdominal wall [24, 25].
To date, no systematic preclinical evaluation has assessed the feasibility of a suprapubic extraperitoneal approach using the Da Vinci SP system. In line with the IDEAL framework for procedural innovation [26], this study explores the preclinical development of a novel suprapubic single-port eTEP technique (SP2 eTEP) for abdominal wall surgery. The study evaluates anatomical reach, procedural feasibility, and skills acquisition in human cadaveric models and porcine live-tissue training, laying the groundwork for future clinical implementation.
Methodology
This preclinical study was designed in accordance with the IDEAL framework for surgical innovation, specifically addressing Stage 1 (Idea) and Stage 2a (Development) (Figure 1) [26]. The study consisted of three components [1]: dry-lab training and technical feasibility assessment [2], cadaveric procedural development, and [3] porcine model skills training.
FIGURE 1
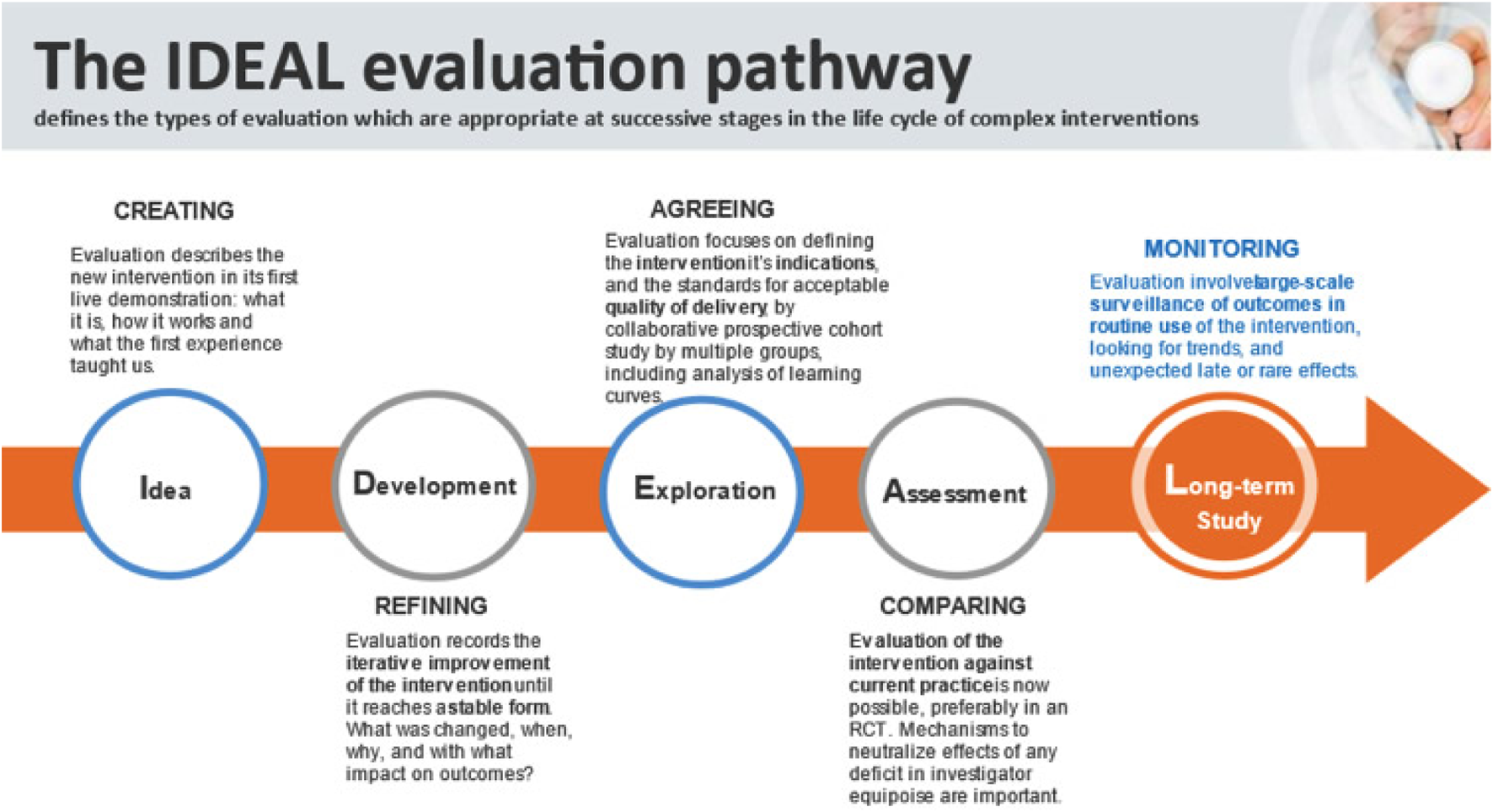
The IDEAL evaluation pathway for surgical innovation [26].
Dry-Lab Training and Technical Feasibility (IDEAL Stage 1: Idea)
Initial exploration of the SP robotic system was conducted with SimNow SP simulator training and with a dry-lab training environment, using a Dietz’ silicone abdominal wall model. The primary objective was to assess the range of motion (ROM) and reach of the SP instruments when introduced via the SP access port and metal cannula through a suprapubic trajectory. Reach to the upper midline (toward the xiphoid) was of particular interest, given its critical importance in enabling ventral hernia and diastasis repairs through a single low incision. The articulating capabilities of the SP system, as well as spatial constraints imposed by port placement, were systematically evaluated to inform subsequent procedural design.
Cadaveric Procedural Development (IDEAL Stage 2a: Development)
A series of cadaveric dissections (n = 3) was undertaken to develop and refine the surgical steps of a SP2 eTEP approach. Dissections included preperitoneal and retrorectus plane development, midline plication for diastasis recti, and bilateral component separation using transversus abdominis release (TAR). Both suprapubic and transumbilical access points were explored to evaluate working angles, instrument triangulation, and the feasibility of completing a full bilateral TAR using the SP system.
Porcine Model Skills Training (IDEAL Stage 2a: Development)
To consolidate SP-specific skills and validate procedural flow under live-tissue conditions, a structured training curriculum was implemented and performed using the previously described SPIRIT porcine model (n = 1) [27]. This model facilitates extraperitoneal dissection and mesh placement with anatomical and technical relevance to human abdominal wall surgery.
Results
Description of the DaVinci SP System
The Da Vinci SP Surgical System (Intuitive Surgical Inc., Sunnyvale, California) was used for all preclinical phases. It comprises a surgeon console with dual 3D-HD displays, a patient-side cart featuring a single robotic arm with four instrument drives (one 12 × 9 mm articulating endoscope and three 6-mm EndoWrist instruments), and a vision cart. All instruments are deployed through a 25 mm SP access port or metal cannula, enabling multi-directional manipulation and a nearly 360° working range within confined anatomical spaces [28].
Description of the DaVinci SP Access Ports
The Da Vinci SP access port is a soft-tissue-compatible, balloon-based port designed to accommodate the SP robotic system’s single-arm architecture. It comprises a flexible introducer with a central lumen of approximately 25 mm, through which a multi-channel cannula is inserted.
The port features:
• An integrated balloon cuff, which is inflated once positioned to maintain port fixation and pneumopreperitoneum during surgery.
• A collapsible housing that, when deflated, enhances instrumental reach and range of motion.
• Low-profile external design, reducing the risk of arm-collision with patient limbs in caudal docking configurations.
In contrast, the rigid metal cannula is a non-collapsible alternative that provides slightly longer straight-line reach but reduced flexibility due to its fixed geometry. It may limit articulation in tight cranial regions unless sufficient working space is developed.
Both systems are compatible with the SP system’s Cobra camera mode, relocation function, and remote center control, which allow for dynamic adjustment of angles and positions during complex abdominal wall procedures.
Dry-Lab Training and Technical Feasibility
The training pathway of robotic assisted surgery traditionally includes preclinic skills development using Da Vinci simulator training (SimNow). For the Da Vinci SP system similar SimNow models on the simulator are available to provide essential training on SP-specific features such as the Cobra camera mode and relocation function.
In May 2025, a dry-lab testing using a Dietz’ silicone abdominal wall model to explore the effective reach and range of motion (ROM) of instruments in various configurations was conducted. Both the standard metal cannula and SP small access port were evaluated in their expanded and collapsed states (Figure 2). Maximum straight and articulated reach were measured from the skin to the instrument tip (Table 1).
FIGURE 2

Ex vivo exploration performed using a Dietz’ silicone abdominal wall model to determine the ROM and reach of the instruments using the Da Vinci SP robotic system either with a metal cannula or with the SP small access port. (courtesy of Prof Ulrich Dietz from Olten, Switzerland).
TABLE 1
| Parameter | Overall instrument length | Metal Cannula | Access Port (Expanded) | Access Port (Collapsed) |
|---|---|---|---|---|
| Maximum Reach (cm) with straight instrument | 55 | 29 | 22 | 28 |
| Maximum Reach (cm) with Articulated instrument | 53 | 27 | 20 | 26 |
Ex vivo exploration performed using a Dietz’ silicone abdominal wall model to determine the ROM and reach of the instruments using the Da Vinci SP robotic system either with a metal cannula or with the SP small access port. (courtesy of Prof Ulrich Dietz from Olten, Switzerland).
Cadaveric Procedural Development
Initial Lab (January 2022)
A procedure development lab was conducted to assess the feasibility of a suprapubic single-port extended totally extraperitoneal (SP2 eTEP) approach for ventral extraperitoneal abdominall wall surgery. The cadaver was placed in dorsal decubitus with the table flexed 15° and a head-down tilt. A 2.7 cm horizontal incision was made 2 cm above the pubic bone to access the preperitoneal space via a “mini-Pfannenstiel” technique. Blunt dissection enabled creation of a preperitoneal working space to introduce the metal SP cannula or small access port (Figure 3). Initial exploration using the metal SP cannula allowed successful dissection of the retrorectus space. However, articulation of the instrument elbows was limited until space was developed cranially. Swapping to the SP small access port improved articulation and maneuverability, especially in the cranial third of the retrorectus dissection. Switching between “camera above” and “camera below” views demonstrated that “camera below” in Cobra mode was optimal for anterior wall procedures (Figure 4).
FIGURE 3
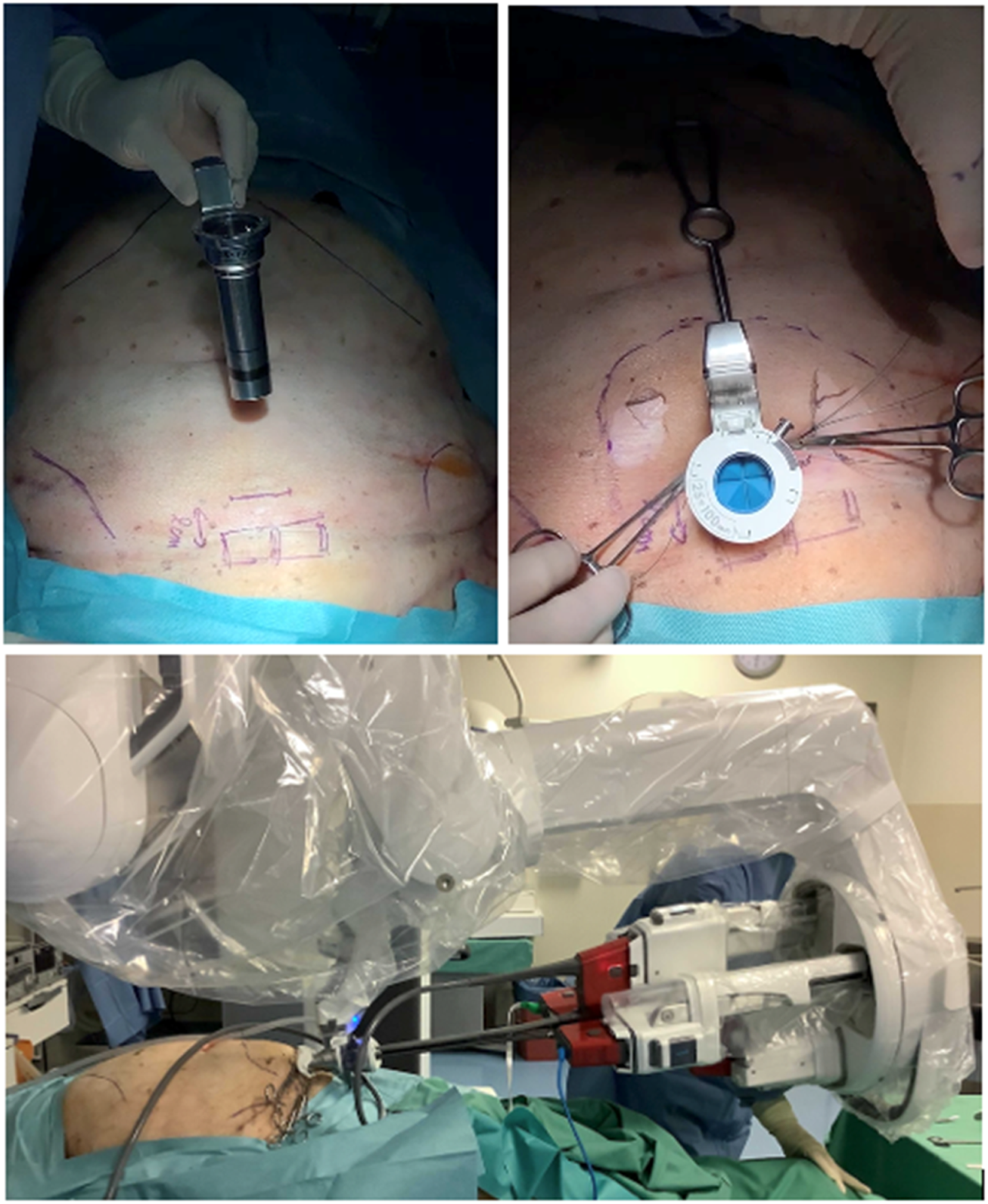
Cadaveric development lab using the metal SP cannula in a suprapubic position for an extraperitoneal retrorectus dissection (SP2 eTEP).
FIGURE 4
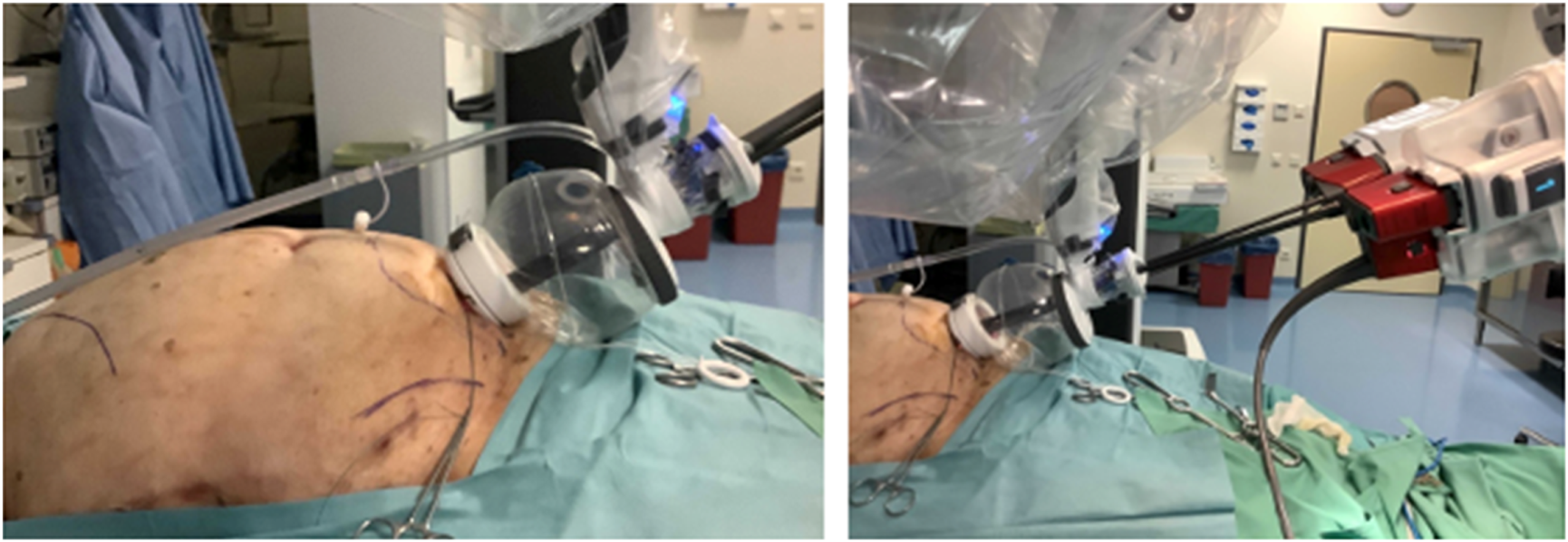
Cadaveric development lab using the SP small access port in a suprapubic position with a camera below position for an extraperitoneal retrorectus dissection (SP2 eTEP).
To increase reach near the xiphoid during midline diastastis closure and TAR near the diafragm, the balloon of the SP access port was collapsed, yielding improved access without trocar displacement (Figure 5). Suturing of the posterior rectus sheath and TAR near the diaphragm were feasible (Figure 6). Mesh insertion was easily performed via the SP assist port.
FIGURE 5
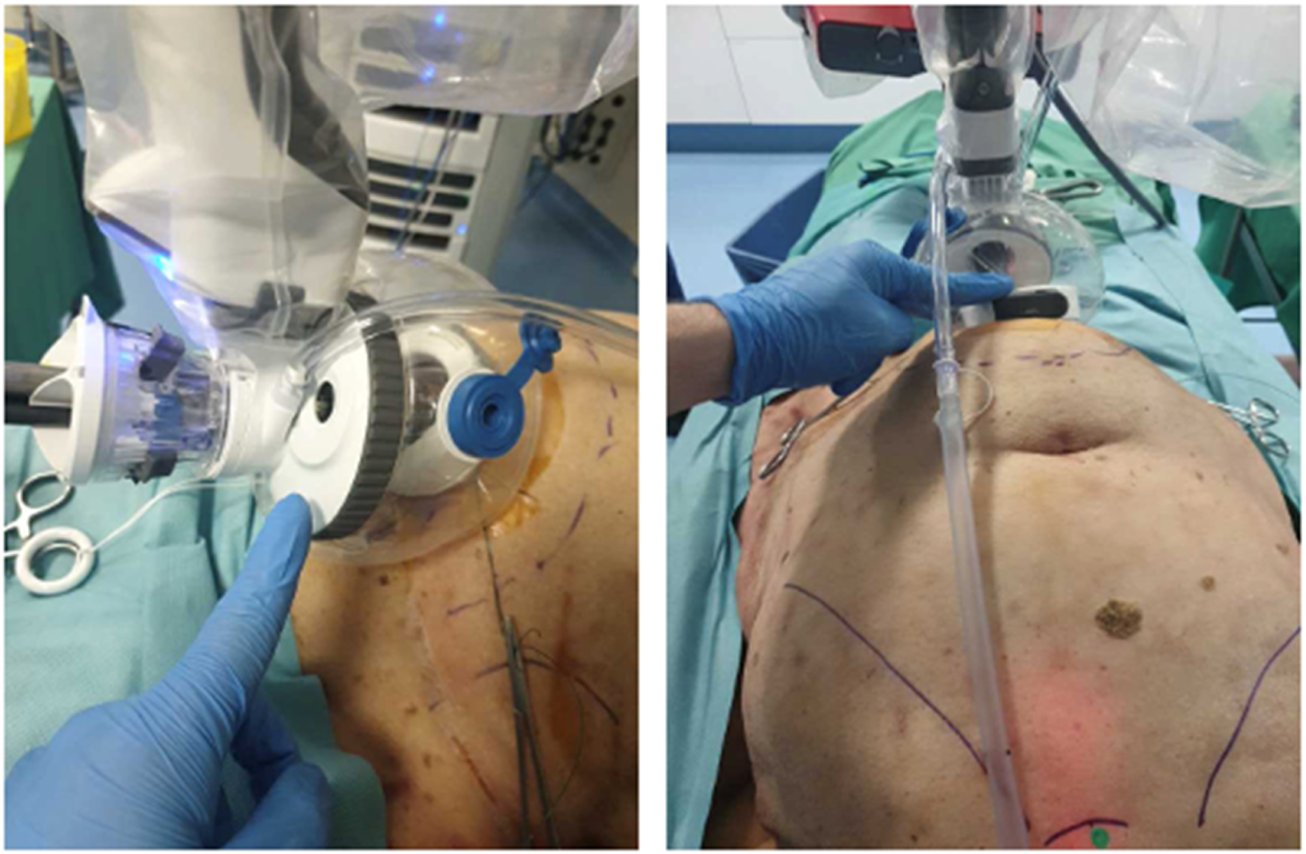
Cadaveric development lab using the SP small access port in a suprapubic position for extraperitoneal retrorectus dissection (SP2 eTEP). The access port is collapsed for maximal ROM and instrument reach cranially.
FIGURE 6
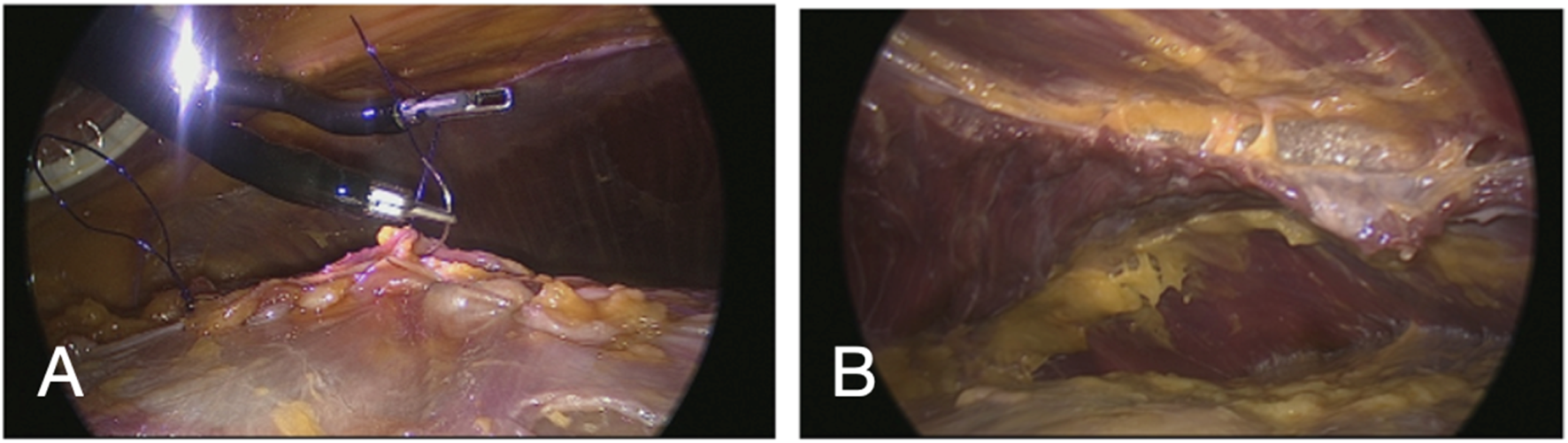
Laparoscopic view with a regular laparoscope through an extra observation trocar during a cadaveric development lab using the SP small access port in a suprapubic position for extraperitoneal retrorectus dissection (SP2 eTEP). Suturing posterior fascia (A) and view of TAR dissection achieved with dissection behind the diaphragm above the watershed fat (B).
Follow-Up Lab (January 2024)
Following CE mark approval for general surgery in Europe, a second cadaveric lab tested software upgrades including the customized remote center (CRC) and pitch limit features. These allowed for safer manipulation without a metal trocar and minimized risk of arm collision with the patient’s lower extremities. The retrorectus dissection, midline closure, and mesh placement were successfully repeated.
To further test system flexibility, the SP access port was relocated to the umbilicus, allowing a successful bilateral TAR without redocking, thanks to the relocation function and nearly 360° workspace (Figures 7, 8).
FIGURE 7
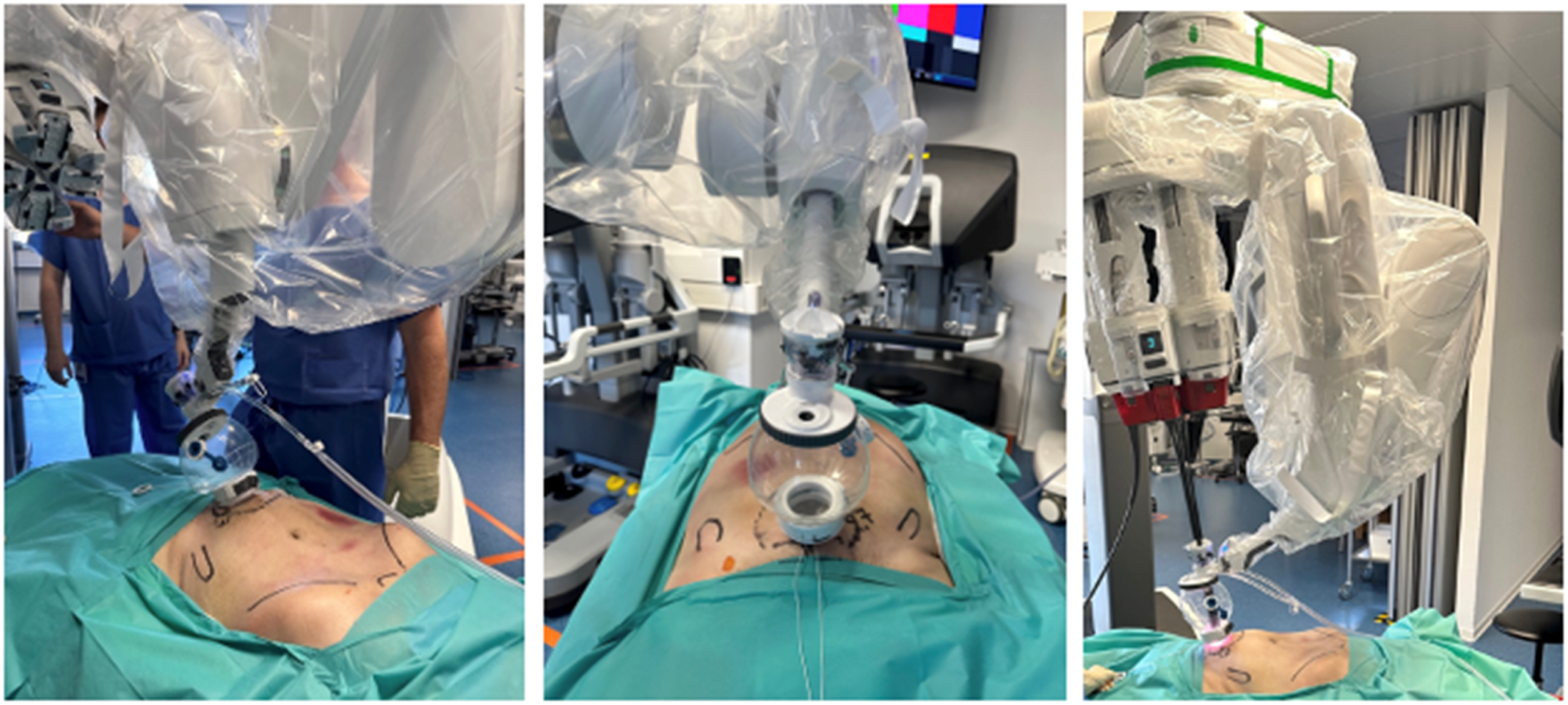
Cadaveric development lab using the SP small access port in a suprapubic position with a camera below position for extraperitoneal retrorectus dissection (SP2 eTEP).
FIGURE 8
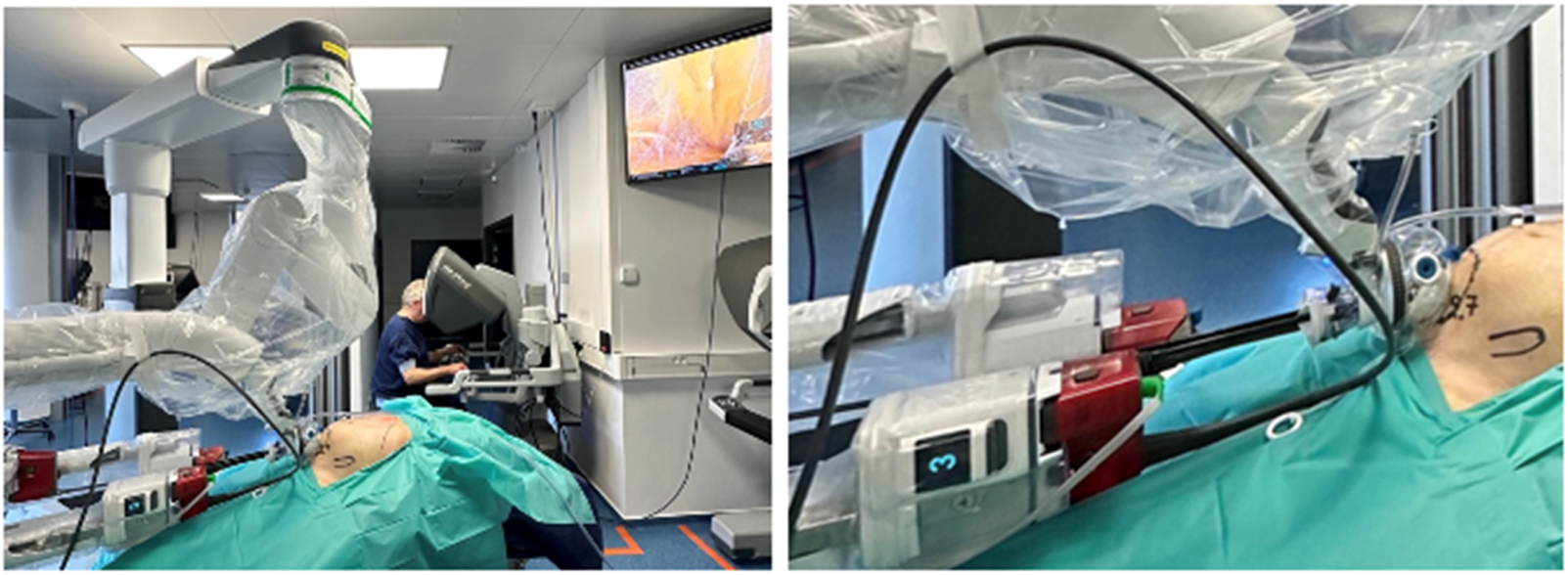
Cadaveric development lab using the SP small access port in a suprapubic position with a camera below position and “collapsed Access Port Balloon” working close to the Pitch limit during the most cranial part of the extraperitoneal retrorectus dissection (SP2 eTEP).
Porcine Model Skills Training
To complement simulator-based SP system training (SimNow), a skills development lab was conducted using the SPIRIT porcine model. This live-tissue model on anesthetized pigs, included in the Da Vinci’s TR300 training curriculum for DaVinci X en Xi system, facilitates extraperitoneal dissection, suturing and mesh placement with anatomical and technical relevance to human abdominal wall surgery [27]. Prior to the clinical use of the Da Vinci SP system, a porcine model skills training lab was performed, exploring the specific features of the SP system while performing a transabdominal preperitoneal repair in the groin (Figure 9). No intraoperative or postoperative complications were observed during the porcine model procedures. As per the standardized training protocol, animal were euthanized under general anesthesia at the end of the session, in full compliance with ethical guidelines.
FIGURE 9
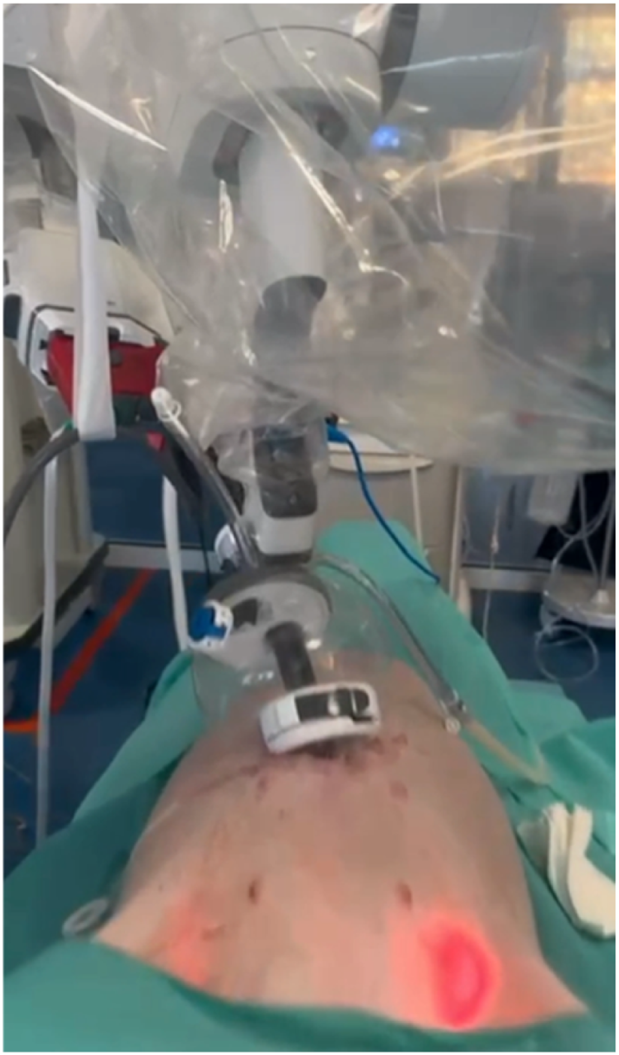
Porcine model skills development using the Da Vinci SP system and the SP small access port for a transabdominal preperitoneal mesh repair in the groin using to the SPIRIT model.
Cadaveric Standardization and SCOLA Exploration (March–April 2025)
Based on this experience, a procedural standardization lab was conducted in April 2025. In addition to SP2 eTEP, we explored the feasibility of performing a SubCutaneous OnLay endoscopic Approach (SCOLA) for diastasis and onlay mesh repair using the SP system. The SP small access port allowed stable maintenance of the subcutaneous working space without leakage. Dissection was followed by a preperitoneal approach and plication of the diastasis using barbed sutures in an Inan inverting (Connell-type) fashion, avoiding midline bulging post-repair (Figure 10).
FIGURE 10
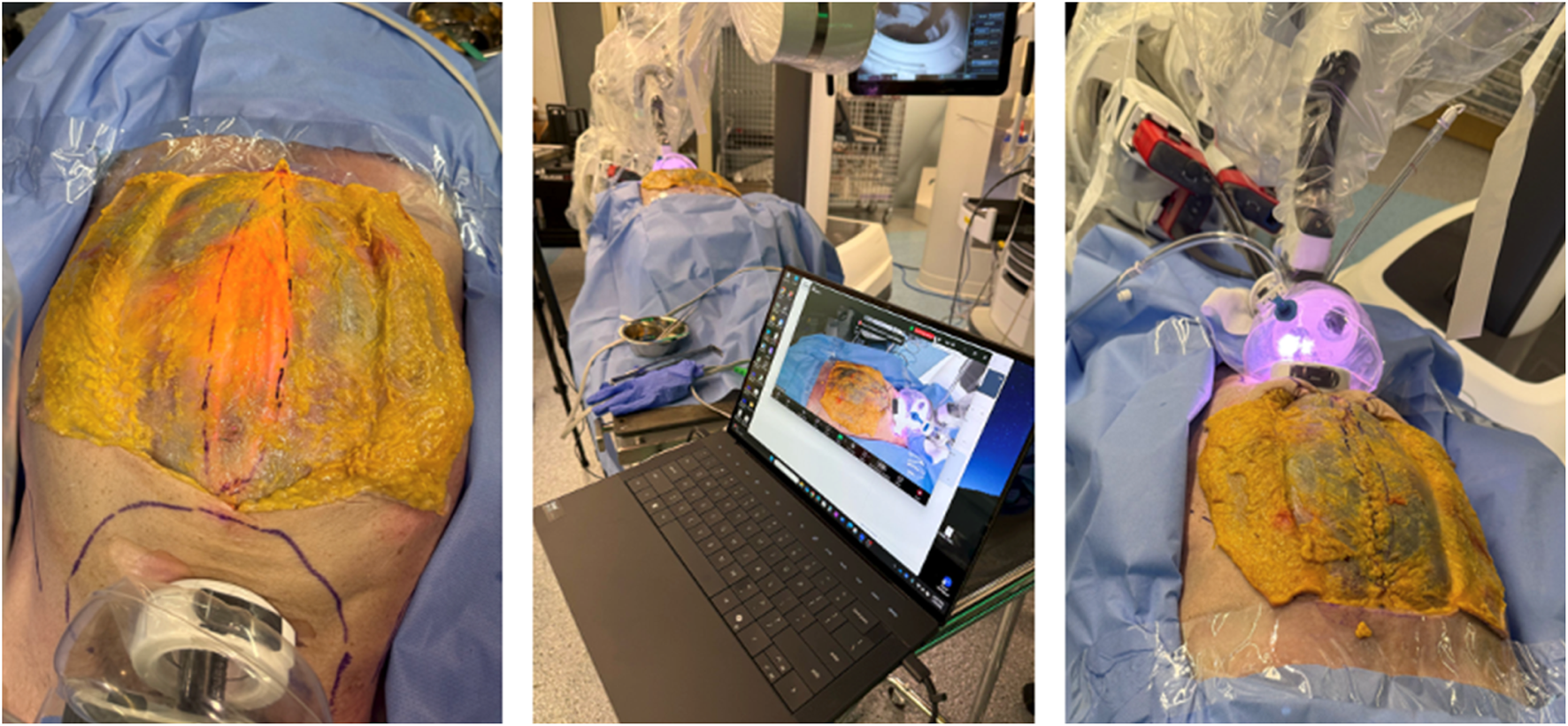
Cadaveric development lab using the SP small access port in a suprapubic position for extraperitoneal retrorectus dissection (SP2 eTEP). The skin and subcutaneous fat has been dissected to expose the anterior abdominal wall and visualize the diastasis plication using the “Inan stitch”.
These results collectively validated the SP2 eTEP technique as feasible and adaptable, and identified the SP system as a promising platform for single-incision ventral abdominal wall surgery.
Discussion
This preclinical study aimed to explore and standardize the use of the Da Vinci SP robotic system for ventral abdominal wall surgery through a suprapubic approach—termed the SP2 eTEP technique. In alignment with IDEAL stage 1 (Idea) and stage 2 (Development) [26], we evaluated system capabilities, instrument reach, procedural steps, and training pathways necessary for safe clinical implementation.
Our dry-lab assessments and cadaveric procedure development sessions demonstrated that the Da Vinci SP platform provides sufficient range of motion and anatomical access to perform simple and complex ventral hernia repairs, including retrorectus mesh placements, preperitoneal dissections, and subcutaneous (SCOLA/ENDOR) reconstructions. The system’s single-arm architecture and fully articulating instruments allowed nearly 360° access, critical for reaching the xiphoid region through a single suprapubic incision. One of the main challenges during the initial cadaveric development of the SP2 eTEP technique was the limited working space available at the beginning of the dissection, especially when using the rigid metal cannula. Due to the confined preperitoneal environment, there was insufficient room for proper deployment of the SP instruments, significantly impairing triangulation and range of motion during the early steps of retrorectus dissection.
One major insight was the performance benefit offered by the SP small access port compared to the traditional metal cannula. The access port’s collapsibility enabled improved reach during cranial dissections and midline closure near the xiphoid. The reach of the collapsed acces port approximated the reach of the metal cannula while retaining full articulation (Table 1; Figure 2 summarize these findings.) This adaptability was especially important in completing complex reconstructions such as bilateral TAR, which was successfully performed from a centralized umbilical access point.
Skills acquisition using a structured simulation pathway is crucial for robotic proficiency. In our training program, we combined SP-specific simulator modules (SimNow) with the SPIRIT porcine model, a validated platform for robotic TAPP procedures [27]. This hybrid model effectively prepares surgeons for both the unique controls of the SP system and the anatomical subtleties of extraperitoneal dissection.
In anticipation of clinical use, we carefully selected early patient cases involving inguinal hernias with concomitant umbilical defects and ventral hernias with diastasis. This strategy aligns with EHS recommendations to adopt straightforward index procedures when implementing new robotic platforms. Furthermore, this diminishes the risk for port-site incisional hernias, which is a key concern after single-port techniques. In the suprapubic approach, we used a mini-Pfannenstiel incision known for its low hernia recurrence rate, and we extended mesh coverage caudally to mitigate the risk of port-site hernias.
We acknowledge that one of the key concerns with new robotic techniques is the potential for increased cost. Further investigation is warranted to compare the overall cost-effectiveness of SP versus multiport robotic and laparoscopic hernia repair, particularly considering operative time, recovery, and complication rates.
Overall, our findings provide a strong foundation for clinical translation of the SP2 eTEP approach. This article marks the completion of our preclinical IDEAL phase, and it serves as the basis for an upcoming prospective case series evaluating safety, reproducibility, and short-term outcomes of ventral hernia repair using the Da Vinci SP system. We believe this next step is essential to validate the clinical utility of the SP2 eTEP technique and refine its indications for broader adoption.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by MedCure. All anatomical specimens provided by MedCure are obtained with the legal consent of donors in accordance with the Uniform Anatomical Gift Act (UAGA) as well as all applicable state and federal laws and regulations. Such consent is specific to specimen procurement and distribution. MedCure abides by Title 45, Part 46 of the U.S. Code of Federal Regulations, ensuring the protection of donor confidentiality and identity.
Author contributions
AD wrote the manuscript; FB wrote the results and structured the tables; FM: idea conceptualization and reviewed the whole manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Henriksen NA Montgomery A Kaufmann R Berrevoet F East B Fischer J et al Guidelines for Treatment of Umbilical and Epigastric Hernias from the European Hernia Society and Americas Hernia Society. Br J Surg (2020) 107(3):171–90. 10.1002/bjs.11489
2.
Henriksen NA Jorgensen LN Friis-Andersen H Helgstrand F , Danish Hernia Database. Open versus Laparoscopic Umbilical and Epigastric Hernia Repair: Nationwide Data on Short- and Long-Term Outcomes. Surg Endosc (2022) 36(1):526–32. 10.1007/s00464-021-08312-5
3.
LeBlanc KA Booth WV . Laparoscopic Repair of Incisional Abdominal Hernias Using Expanded Polytetrafluoroethylene: Preliminary Findings. Surg Laparosc Endosc (1993) 3(1):39–41.
4.
Muysoms FE Bontinck J Pletinckx P . Complications of Mesh Devices for Intraperitoneal Umbilical Hernia Repair: A Word of Caution. Hernia (2011) 15(4):463–8. 10.1007/s10029-010-0692-x
5.
Chowbey PK Sharma A Khullar R Soni V Baijal M . Laparoscopic Ventral Hernia Repair with Extraperitoneal Mesh: Surgical Technique and Early Results. Surg Laparosc Endosc Percutan Tech (2003) 13(2):101–5. 10.1097/00129689-200304000-00008
6.
Sauerland S Walgenbach M Habermalz B Seiler CM Miserez M . Laparoscopic versus Open Surgical Techniques for Ventral or Incisional Hernia Repair. Cochrane Database Syst Rev (2011)(3):CD007781. 10.1002/14651858.CD007781.pub2
7.
Ramakrishna HK Lakshman K . Intra Peritoneal Polypropylene Mesh and Newer Meshes in Ventral Hernia Repair: What EBM Says?Indian J Surg (2013) 75(5):346–51. 10.1007/s12262-012-0743-x
8.
Tran E Sun J Gundara J . Systematic Review of Robotic Ventral Hernia Repair with Meta-Analysis. ANZ J Surg (2024) 94(1–2):37–46. 10.1111/ans.18822
9.
Christoffersen MW Andresen K Perregaard H Henriksen NA . Robotic Surgical Procedures for Ventral Hernia Repair. J Abdom Wall Surg (2025) 4(February):14212–0. 10.3389/jaws.2025.14212
10.
Belyansky I Daes J Radu VG Balasubramanian R Reza Zahiri H Weltz AS et al A Novel Approach Using the Enhanced-View Totally Extraperitoneal (eTEP) Technique for Laparoscopic Retromuscular Hernia Repair. Surg Endosc (2018) 32(3):1525–32. 10.1007/s00464-017-5840-2
11.
Claus C Cavazzola L Malcher F . SubCutaneous OnLay Endoscopic Approach (SCOLA) for Midline Ventral Hernias Associated with Diastasis Recti. Hernia (2021) 25(4):957–62. 10.1007/s10029-021-02465-x
12.
Vierstraete M De Troyer A Pletinckx P Hermie E Muysoms F . Lateral Single-Dock Robot-Assisted Retro-Rectus Ventral Hernia Repair (rTARUP/rTARM): Observational Study on Long-Term Follow-Up. J Robot Surg (2025) 19(1):84. 10.1007/s11701-025-02243-2
13.
Brucchi F Boni L Cassinotti E Baldari L . Short-Term Outcomes of Minimally Invasive Endoscopic Onlay Repair for Diastasis Recti and Ventral Hernia Repair: A Systematic Review and Meta-Analysis. Surg Endosc (2025) 39(3):1490–500. 10.1007/s00464-025-11555-1
14.
Malcher F Lima DL Lima RNCL Cavazzola LT Claus C Dong CT et al Endoscopic Onlay Repair for Ventral Hernia and Rectus Abdominis Diastasis Repair: Why So Many Different Names for the Same Procedure? A Qualitative Systematic Review. Surg Endosc (2021) 35(10):5414–21. 10.1007/s00464-021-08560-5
15.
Bracale U Corcione F Neola D Castiglioni S Cavallaro G Stabilini C et al Transversus Abdominis Release (TAR) for Ventral Hernia Repair: Open or Robotic? Short-Term Outcomes from a Systematic Review with Meta-Analysis. Hernia (2021) 25(6):1471–80. 10.1007/s10029-021-02487-5
16.
Belyansky I Reza Zahiri H Sanford Z Weltz AS Park A . Early Operative Outcomes of Endoscopic (eTEP Access) Robotic-Assisted Retromuscular Abdominal Wall Hernia Repair. Hernia (2018) 22(5):837–47. 10.1007/s10029-018-1795-z
17.
Chelliah DE Schroeder AD Johnson CJ Boardman J Florin JL . Outcomes from 306 Consecutive Robotic Ventral Hernia Repairs in the Community Setting. J Robot Surg (2025) 19(1):32. 10.1007/s11701-024-02201-4
18.
Dietz UA Kudsi OY Garcia-Ureña M Baur J Ramser M Maksimovic S et al Robotic Hernia Repair III. English Version: Robotic Incisional Hernia Repair with Transversus Abdominis Release (R-TAR). Video Report and Results of a Cohort Study. Chirurg. (2021) 92(August):28–39. 10.1007/s00104-021-01500-y
19.
Radu VG Cucu DT . The eTEP/eTEP-TAR Repair of Ventral Hernias a Study from One Center/One Surgeon—The First Five Years of Experience. J Abdom Wall Surg (2024) 3(April):12796–6. 10.3389/jaws.2024.12796
20.
Arias-Espinosa L Claus CM Malcher F Valenzuela Alpuche HA . Robotic Preperitoneal Extended Totally Extraperitoneal (R-PeTEP) Technique Description for Ventral Hernia Repair: Preliminary Results. Updates Surg (2024) 76(7):2715–22. 10.1007/s13304-024-02002-2
21.
Pini R Mongelli F Iaquinandi F Gaffuri P Previsdomini M Cianfarani A et al Switching from Robotic-Assisted Extended Transabdominal Preperitoneal (eTAPP) to Totally Extraperitoneal (eTEP) Hernia Repair for Umbilical and Epigastric Hernias. Sci Rep (2024) 14(1):1800–8. 10.1038/s41598-024-52165-6
22.
Olivier F Abasbassi M Geers J . Robotic Retromuscular Abdominal Wall Repair Using an Inverted TEP (iTEP) Approach: Surgical Technique and Short-Term Outcomes. Langenbeck’s Arch Surg (2022) 407(5):2177–86. 10.1007/s00423-022-02561-1
23.
Pini R Di Giuseppe M Toti JMA Mongelli F Marcantonio M Spampatti S et al Robot-Assisted Treatment of Epigastric Hernias with a Suprapubic Approach. Surg Laparosc Endosc Percutan Tech (2021) 31(5):584–7. 10.1097/SLE.0000000000000941
24.
Celotto F Ramacciotti N Mangano A Danieli G Pinto F Lopez P et al Da Vinci Single-Port Robotic System Current Application and Future Perspective in General Surgery: A Scoping Review. Surg Endosc (2024) 38(9):4814–30. 10.1007/s00464-024-11126-w
25.
Bianco FM Dreifuss NH Chang B Schlottmann F Cubisino A Mangano A et al Robotic Single-Port Surgery: Preliminary Experience in General Surgery. Int J Med Robot Comput Assist Surg (2022) 18(6):e2453. 10.1002/rcs.2453
26.
Cook JA McCulloch P Blazeby JM Beard DJ Marinac-Dabic D Sedrakyan A et al IDEAL Framework for Surgical Innovation 3: Randomised Controlled Trials in the Assessment Stage and Evaluations in the Long Term Study Stage. BMJ (2013) 346(box 1):f2820–5. 10.1136/bmj.f2820
27.
Vierstraete M Muysoms F . Standardized Porcine Integrated Robotic Inguinal Hernia Training: The SPIRIT Model. Surg Endosc (2024) 38(8):4229–35. 10.1007/s00464-024-10932-6
28.
Lai A Luoyia G di Meo NA Crivellaro S . Single-Port Robotic Surgery: General Principles and Troubleshooting. J Endourol (2022) 36(September):25–8. 10.1089/end.2022.0313
Summary
Keywords
robotic surgery, single port, suprapubic eTEP, abdominal wall surgery, preclinical research
Citation
De Troyer A, Brucchi F and Muysoms F (2025) Using the Da Vinci Single Port Robotic Platform in Abdominal Wall Surgery: Preclinical Exploration of a Suprapubic Single Port Extraperitoneal Approach (SP2 eTEP). J. Abdom. Wall Surg. 4:15230. doi: 10.3389/jaws.2025.15230
Received
06 July 2025
Accepted
25 September 2025
Published
03 October 2025
Volume
4 - 2025
Updates
Copyright
© 2025 De Troyer, Brucchi and Muysoms.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annabelle De Troyer, annabelle.detroyer@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.