Abstract
Dystonia causes involuntary, patterned movements and posturing, often leading to disability, pain, and reduced quality-of-life. Despite standard treatments such as botulinum toxin (BoNT) injections, oral medications, and deep brain stimulation therapy, many patients continue to experience persistent symptoms. There is growing evidence supporting the use of rehabilitation-based therapies in the management of certain forms of dystonia. This review summarizes the current body of evidence, which primarily focuses on cervical dystonia (CD) and task-specific dystonia (TSD). The greatest therapeutic potential appears to lie in using these interventions as adjuncts to BoNT therapy. In CD, physical therapy has shown effectiveness when aimed at reducing overactivity in the affected neck muscles through techniques such as stretching, massage, and biofeedback. Concurrently, strengthening the opposing muscle groups helps promote improved posture, reduce pain, and enhance range of motion. In TSD, many studies applied splinting of unaffected body parts (sensory-motor retuning) to encourage adaptive retraining of affected body parts (principles of constraint-induced movement therapy), or alternatively restricting movements of affected body parts to promote sensory reorganization. Although there is high risk of bias, neuroplasticity-based strategies like motor and sensorimotor training appear to be promising for TSD. Use of kinesiotaping, vibrotactile stimulation, TENS, and orthotics can help modify movement patterns, while biofeedback can reinforce and sustain motor control improvements. Emerging evidence for functional dystonia supports the role of multimodal approach, combining PT with cognitive behavioral therapy or mind-body strategies. The focus is movement retraining to shift attention away from abnormal movements and restore confidence in normal movement to improve outcomes. Regardless of dystonia type, individualized therapy plans are essential. Home-based exercises play a critical role in maintaining the gains achieved during supervised sessions, supporting ongoing progress, and preventing regression.
Introduction
Dystonia, the third most common movement disorder, is characterized by involuntary, patterned movements and abnormal posturing. Dystonia is classified as focal as in cervical dystonia (CD), blepharospasm, laryngeal dystonia (LD) or limb dystonia, segmental, multifocal, hemi- or generalized (trunk with or without leg involvement) based on the number of body regions involved [1]. Focal forms of dystonia can impair activities of daily living such as feeding, dressing, reading, driving, watching television, and engaging in social interactions whereas generalized forms of dystonia can severely impair mobility, self-care, and the ability to maintain employment [2]. All forms of dystonia, whether focal or generalized are frequently associated with chronic pain, fatigue, depression, anxiety, and social withdrawal. The overall burden on quality of life (QoL) is strongly influenced by the distribution and severity of dystonia, with more widespread or intense symptoms leading to greater functional impairment and disability [3, 4].
A significant number of dystonia patients continue to experience symptoms despite receiving standard treatments such as botulinum toxin (BoNT) injections, oral medications, or surgical therapies such as deep brain stimulation (DBS) [5]. However, the effectiveness of these treatments has been reported to vary widely depending on the underlying disease and the condition of the patient. Systematic reviews have shown that the quality of evidence supporting the efficacy of BoNT treatment is strongest for CD and blepharospasm, primarily due to the availability of multiple high-quality randomized controlled trials (RCTs). In contrast, evidence for its use in laryngeal and limb dystonia, while promising, remains more limited due to smaller study sizes and methodological variability [6, 7]. Patients with generalized dystonia may experience limited functional improvement despite accurate and successful DBS electrode implantation in appropriate brain targets [8].
Rehabilitation for dystonia focuses on improving functional abilities, reducing pain, and enhancing QoL for individuals affected by the disorder [9]. The World Health Organization defines rehabilitation as a set of interventions designed to optimize functioning and reduce disability in individuals with health conditions in interaction(s) with their environment [10]. Physical therapy (PT) involves tailored exercises, neuromuscular re-education and manual techniques aimed at improving range of motion, strength, and postural control to improve balance and functional mobility [11]. Occupational therapy (OT) focuses on helping patients manage daily activities (e.g., writing, dressing) by providing adaptive tools or strategies to reduce functional impairments [12]. In the context of TSD, the use of adaptive tools, such as specialized writing aids or customized splints, can help patients manage tasks more effectively [9, 13]. Speech therapy aids in improving communication, swallowing, and breathing, particularly in laryngeal and oromandibular dystonias [13]. Cognitive-Behavioral Therapy (CBT) assists in addressing the emotional burden of dystonia, such as depression, anxiety, and social isolation [14]. There is limited data summarizing the evidence on the role of rehabilitation in dystonia, as these interventions are both under-investigated and underutilized.
Some previous study groups that had reviewed rehabilitation strategies in dystonia categorized data based on underlying shared theoretical foundations, aiming to identify commonalities in therapeutic approaches across different types of dystonia [13, 15]. While synthesizing evidence based on theoretical frameworks can help guide future clinical research and enhance mechanistic understanding, a significant limitation, acknowledged by these researchers, is that many studies incorporated multiple treatments all at the same time without isolating the effects of individual therapy. We therefore adopted a different approach for this review when categorizing the study data. We classified studies into the following four groups (1) Use of Multimodal or Combination Strategies. In this category, studies combining physical and/or behavioral interventions with pharmacological treatments, such as BoNT injections, or neuromodulation approaches like transcranial direct current stimulation (tDCS), or transcranial magnetic stimulation (TMS) were included. Many studies utilized multimodal combination approaches, for example, integrating physical exercise programs with botulinum therapy or, in the case of functional dystonia, combining psychotherapy, physical therapy, and occupational therapy [16–44]. (2) Use of Exercise/Stretching/Relaxation/Biofeedback therapy. In this category, studies focusing exclusively on physical interventions or exercise programs were included. (3) Use of Adaptive Aids or External Devices (that are potentially wearable) such as Kinesiotape, Splints, Vibrotactile stimulation, Orthotic device, Transcutaneous Electrical Stimulation (TENS) and Functional Electrical Stimulation (FES). In this category, we included studies specifically employing adaptive devices as the key component for managing dystonia. (4) Use of Behavioral or Psychotherapy. In this category, we examined studies that centered solely on behavioral interventions such as CBT or mind-body programs. Our review aims to guide clinical providers in making informed referrals for rehabilitation that may benefit their patients based on current evidence (whether it should be referrals for PT, OT, speech therapy, psychotherapy, or multiple disciplines). Additionally, it plans to offer researchers insights into future directions for designing more rigorous studies.
Methods
We searched PubMed in October 2024 to review literature published on this topic from 1976 to 2024 using various combinations of keywords such as “focal dystonia,” “segmental dystonia,” or “generalized dystonia,” combined with terms related to rehabilitation interventions such as “exercise-based interventions”, or “behavioral interventions”. As an example, we searched for articles related to focal dystonia and exercise interventions using the following keywords and combinations: (“Focal dystonia” [Mesh] OR “focal dystonia” [Title/Abstract]) AND (“rehabilitation” [Mesh] OR “rehabilitation” [Title/Abstract] OR “exercise” [Mesh] OR “exercise” [Title/Abstract] OR “behavioral intervention” [Mesh] OR “behavioral intervention” [Title/Abstract]).
Inclusion criteria consisted of: (1) Isolated CD, blepharospasm, cranial dystonia, Meige syndrome, LD, limb dystonia, generalized dystonia, functional or psychogenic dystonia) treated with one or more rehabilitation strategy. CD is the most common form of adult-onset dystonia, impairing voluntary head control, with presentations ranging from pronounced postural deviations with phasic components to minimal postural changes accompanied primarily by head tremor [45]. Patients with blepharospasm experience involuntary eyelid muscle spasms, leading to excessive blinking or sustained eyelid closure, which can result in functional blindness [46]. Task-specific dystonia (TSD) is a type of focal dystonia characterized by involuntary muscle contractions that interfere with highly skilled, repetitive movements practiced or performed over several years (often decades). Common forms include writer’s cramp (WC) and musician’s dystonia (MD), which typically affect pianists, guitarists, and drummers, as well as embouchure dystonia in wind instrument players. However, TSD can impact a wide range of other occupations and activities, including typing, hairdressing, painting, tailoring, dancing, shooting, and sports such as golf or table tennis, where fine motor control and repetitive movement are essential [13]. In LD, involuntary spasms of the vocal cord muscles during speech, results in a strained, strangled, breathy, or shaky voice that significantly impairs communication [47]. LD is a form of TSD, with most individuals experiencing selective impairment during speaking. However, speech production may be relatively spared during whispering or innate vocal behaviors such as laughing, crying, yawning, as well as other upper respiratory functions like coughing and sniffing. In professional singers, after years of vocal performance, dystonia manifests only during singing (referred to as singer’s dystonia) [47]. Functional dystonia is the second most common form of functional movement disorder, characterized by the acute or subacute onset of fixed postures in the limbs, trunk, or face that do not align with the typical symptoms of movement-provoked, position-sensitive, or task-specific dystonia [48]. (2) Use of intervention methods such as exercise training, stretching, relaxation, biofeedback, kinesiotaping, vibrotactile stimulation, immobilization with splints, sensory training, neuromodulation combined with motor training, (3) Prospective design. Exclusion criteria consisted of: (1) Studies involving children (age <10 years), (2) Studies where the primary goal was not to test an intervention but rather to test the mechanism of a specific method or disease mechanism, (3) Studies involving neuromodulation alone without motor or other rehab training, (4) Observational and retrospective studies, reviews, editorials, commentaries or expert opinion, conference proceedings and abstracts, (5) Studies with unclear methods or results, (6) Studies published or data available in languages other than English.
The titles and abstracts of all identified studies were independently reviewed by two researchers (HK and KN). In addition, relevant studies cited in the reference lists or bibliographies of selected articles were also assessed for inclusion. Final decisions regarding the inclusion of publications in the systematic review were made based on the agreement of the two reviewers, in accordance with the predefined inclusion and exclusion criteria. In the following results section, we present the study characteristics for each dystonia condition, identifying key themes based on shared attributes such as study design, intervention type, classification of dystonia, intervention duration, and outcomes. We report statistically significant beneficial effects as “significant improvements,” while qualitative improvements not tested for statistical significance are described as “improvements.” The risk of bias for each study included in this review was assessed using a four-tiered classification scheme followed by the American Academy of Neurology (AAN). The risk of bias for each study included in this review has been measured using a four-tiered classification scheme followed by the AAN with studies rated Class I are judged to have a low risk of bias, Class II is judged to have a moderate risk of bias, Class III, a moderately high risk of bias; and Class IV, a very high risk of bias. The recommendations we provide do not follow the AAN framework, as the available literature is relatively limited. Instead, they are based on a qualitative synthesis of individual study data, considering factors such as the balance of benefits and harms, feasibility, and acceptability.
Results
After screening 232 titles and abstracts, excluding duplicate records and non-English publications, and identifying additional studies from bibliographies, we ultimately selected 72 studies for data extraction (Figure 1).
FIGURE 1
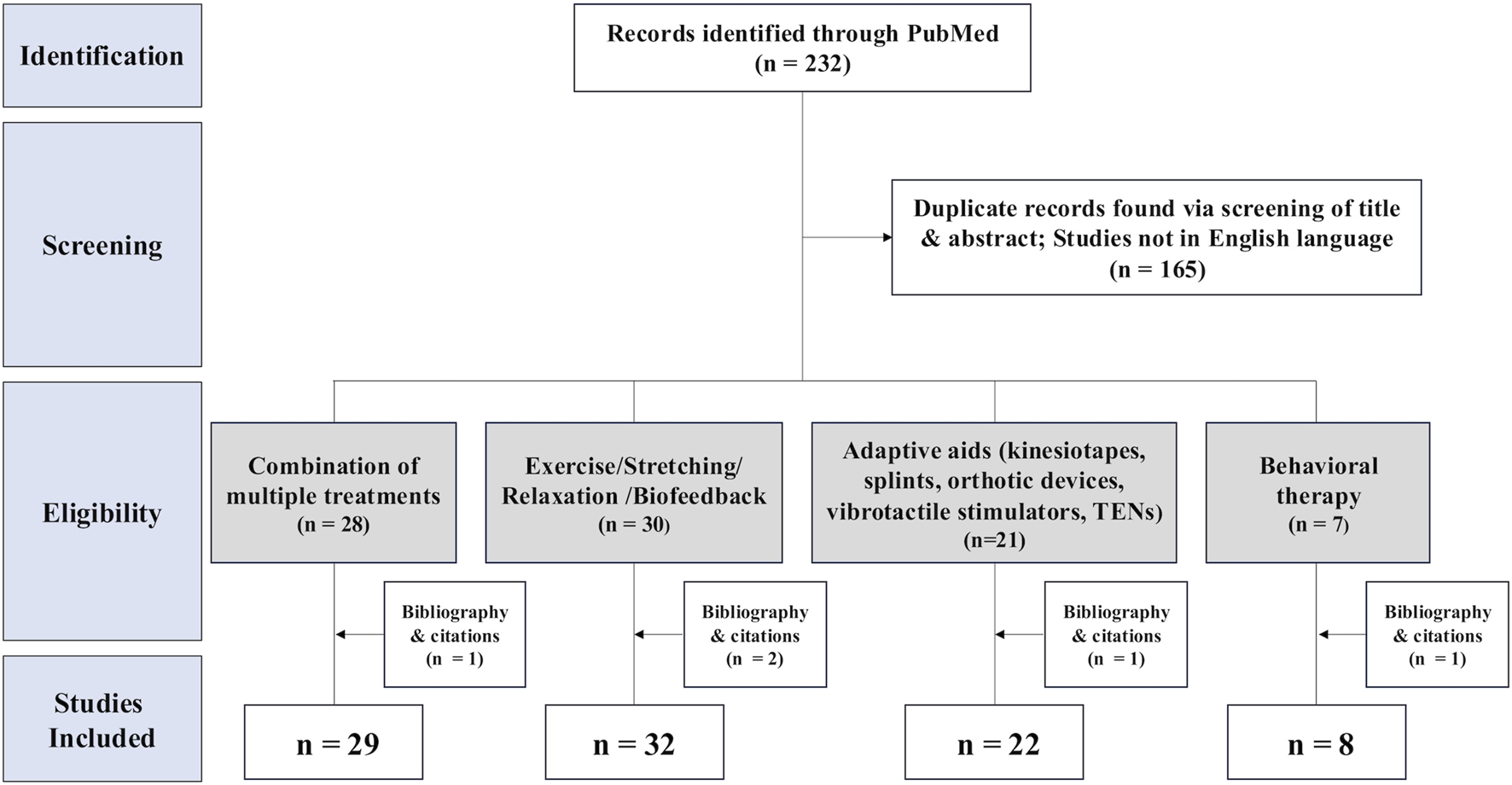
PRISMA flow diagram of study selection process in the systematic review. 232 records were found in the search. Screening excluded 165 reports; 91 studies were categorized into four groups. (1) Combination of multiple treatments (n = 29), (2) Exercise/Stretching/Relaxation/Biofeedback (n = 32), (3) Adaptive aids (kinesiotapes, splints, orthotic devices, vibrotactile stimulators, TENs) and orthotic devices, vibrotactile stimulators, TENs) (n = 22), (4) Behavioral therapy (n = 8).
We identified a range of study designs, including RCTs, case-control studies, before-after studies, and case reports. Significant heterogeneity was observed across studies in terms of interventions, implementation strategies, assessed outcomes, reporting details, and follow-up durations, which varied from one day to four years. Many studies were small-scale, lacked proper control conditions or randomization, and carried a high risk of bias. Despite these limitations, we conducted a qualitative assessment of studies, focusing primarily on those deemed to be of low risk for bias, to inform clinical practice recommendations.
Effects of multimodal or Combination Strategies
We identified 29 studies in this category. Individual study results are presented in Table 1. There were 15 studies on CD, three on Meige syndrome and LD, six on focal limb dystonia (2 WC, 4 MD), and six on functional dystonia. Regarding study design, eight studies were RCTs, including crossover designs, two were non-randomized crossover trials, two employed a pre-post design, one was a case-control study, and one was a case report.
TABLE 1
| Combination of multiple treatments (n = 29) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Study design | Participant characteristics | Intervention | Assessment outcomes | Results | AAN class | ||||
| Active group | Control group | Active | Control | |||||||
| Focal Cervical Dystonia | ||||||||||
| Bleton et al. [16] France | Cross-over | N | 5 | Treatment | Motor training + tDCS | tDCS | TWSTRS (severity) | Significantly greater & longer improvement in dystonia severity | III | |
| M/F | 1/4 | Details | Cerebellar anodal tDCS + activating impaired muscles | |||||||
| Age (y) | 63 (40–79) | Frequency/Duration | 20 min/d, 3–5 d/w, 1w | |||||||
| Dec-Ćwiek. et al. [17] Poland | RCT cross-over | N | 19 | Treatment | Kinesiotaping + BoNT | Sham taping + BoNT & BoNT alone | TWSTRS, CDQ24, subjective) | Significant improvement in QoL No significant difference in dystonia severity | II | |
| M/F | 4/15 | Details | Kinesiotaping applied to shoulder Kinesiotaping initiated 7 d after BoNT | |||||||
| Age (y) | 54 ± 12 | Frequency/Duration | 3 conditions; 12w/condition; 36w | |||||||
| Castagna. et al. [18] Italy | Cross-over | N | 15 | Treatment | Supervised PT + BoNT | BoNT | TWSTRS (severity) | Significant improvement in dystonia severity with addition of PT | III | |
| M/F | 8/7 | Details | Augmented feedback of movement (visual & acoustic) + BoNT PT initiated just after BoNT | |||||||
| Age (y) | 48 ± 9 | Frequency /Duration | BoNT: 2 sessions + Exercise: 18 sessions, 6w, 18w | |||||||
| de Oliveira et al. [28] Brazil | Pre-post | N | 2 | Treatment | Progressive exercises + tDCS | NA | TWSTRS, WCRS (severity, pain) | Improvement in dystonia severity & pain | IV | |
| M/F | 1/1 | Details | Exercises for cervical and trunk muscles + tDCS (2 mA, 20 min, PMC) | |||||||
| Age (y) | 79, 48 | Frequency/Duration | 15 sessions, 3 m | |||||||
| Werner et al. [19] Germany | RCT | N | 18 | Treatment | Supervised PT + BoNT | BoNT | ROM, SF-36, TWSTRS (function, subjective, severity) | Significant improvement in ROM, subjective symptoms, & severity with addition of PT | II | |
| M/F | 3/15 | Details | Reduction of pathological movement patterns PT performed between two BoNT injection sessions | |||||||
| Age (y) | 63 ± 13 | Frequency/Duration | BoNT: 2 sessions + PT: 45 min/session, 2/w, 3 m | |||||||
| Hu. et al. [20] USA | RCT | N | 8 | 8 | Treatment | Supervised and home-based PT + BoNT | BoNT | TWSTRS (severity) | Significant improvement in dystonia severity with addition of PT | II |
| M/F | 4/4 | 5/3 | Details | Stretching, range-of-motion, isometric exercises PT initiated after BoNT on the same day | ||||||
| Age (y) | 64 ± 7 | 67 ± 7 | Frequency/Duration | 15 min/d, 5 d/w, 6 w | ||||||
| van den Dool et al. [27] Netherlands | RCT | N | 48 | 48 | Treatment | Supervised customized PT + BoNT | Supervised regular PT + BoNT | TWSTRS disability (severity) | Both groups significantly improved in dystonia severity | I |
| M/F | 19/29 | 18/30 | Details | Stretching, range-of-motion, passive mobilization, biofeedback PT initiated 2w after BoNT | ||||||
| Age (y) | 59 ± 9 | 57 ± 9 | Frequency/Duration | 1/w, 1y | ||||||
| Stankovic. et al. [25] Serbia | RCT | N | 9 | 4 | Treatment | BoNT + PT supervised clinic-based & home-based | Home based PT | Tsui scale, TWSTRS (severity) | Significantly greater & longer improvement in dystonia severity | II |
| M/F | 11/3 | Details | Exercises, stretching, OT, functional therapy PT initiated 5d after BoNT | |||||||
| Age (y) | 42 ± 5 | Frequency/Duration | BoNT: 1 session + Supervised PT: 5 d/w, 2w + Home PT, 6 m | |||||||
| Bradnam et al. [29] Australia | RCT | N | 16 | Treatment | rTMS + motor training | sham rTMS | TWSTRS, CDQ24 (severity, pain, subjective) | Significant improvement in dystonia severity, pain, subjective symptoms | II | |
| M/F | 6/10 | Details | Intermittent theta-burst stimulation for cerebellum + Motor training for neck | |||||||
| Age (y) | 28–72 | Frequency/Duration | 2s train every 10s, total of 190s or 600 pulses, 10 sessions | |||||||
| Counsell et al. [22] UK | RCT | N | 55 | 55 | Treatment | Specialized supervised PT | Relaxation, exercises to increase ROM, core stability (home-based) | TWSTRS (severity) | Both groups significantly improved in severity | I |
| M/F | 17/38 | 13/42 | Details | Strengthening of underactive muscles, advice about posture, awareness of body position, relaxation of overactive muscles | ||||||
| Age (y) | 55 (13) | 57 (12) | Frequency/Duration | 45 min/session, 1 session/w, 24w | ||||||
| Queiroz et al. [21] Brazil | Case-control | N | 20 | 20 | Treatment | PT with FES + BoNT | BoNT | TWSTRS, SF-36 (severity, subjective) | Significant improvement in ADL & subjective pain | III |
| M/F | 9/11 | 11/9 | Details | Motor learning exercises, kinesiotherapy, FES on antagonist muscles PT initiated 15 d after BoNT | ||||||
| Age (y) | 52 (14) | 50 (12) | Frequency/Duration | 25 min/session, 5 session/w, 4w | ||||||
| El-Bahrawy et al. [26] Egypt | RCT | N | 20 | 20 | Treatment | Exercise therapy & TENS + BoNT | Sham TENS + BoNT | Head posture & ROM, Purdue Peg Board Test (function) | Significant improvement in head posture | II |
| M/F | 13/7 | 12/8 | Details | Stretching, training of voluntary movements, TENS BoNT performed at least 1 m before PT | ||||||
| Age (y) | 32 ± 4 | 32 ± 3 | Frequency/Duration | BoNT: 1 session + PT: 3 sessions/w, 18 sessions; 6w | ||||||
| Tassorelli et al. [23] Italy | RCT cross-over | N | 20 | 20 | Treatment | Exercise therapy + BoNT | BoNT | Tsui scale, TWSTRS, ADL, total pain scale (severity, subjective, pain) | Both groups significantly improved in dystonia severity Pain & ADL significantly improved in exercise group; BoNT effects prolonged | II |
| M/F | 7/13 | 6/14 | Details | Massage, passive myofascial elongation maneuvers, stretching, biofeedback PT initiated just after BoNT | ||||||
| Age (y) | 50 ± 16 | 52 ± 14 | Frequency/Duration | 60–90 min/d, daily, 2w | ||||||
| Ramdharry et al. [24] UK | Case report | N | 1 | Treatment | PT + BoNT | NA | TWSTRS (severity) | Improvement in dystonia severity & increased BoNT effects | IV | |
| M/F | M | Details | Strengthen neck muscles PT initiated just after BoNT | |||||||
| Age (y) | NA | Frequency/Duration | 14 sessions, 6 m | |||||||
| Gildenberg et al. [30] USA | Pre-post | N | 29 | Treatment | Biofeedback training + TENS | NA | Overall Symptoms (subjective) | 4/29 responded to biofeedback training 3/29 responded to TENS | IV | |
| M/F | NA | Details | EMG biofeedback, relaxation, TENS over sternocleidomastoid | |||||||
| Age (y) | NA | Frequency/Duration | Biofeedback training was tried first TENS performed to ineffective patients | |||||||
| Meige Syndrome and Laryngeal Dystonia | ||||||||||
| Cairns et al. [31] Canada | Case report | Type | Meige syndrome | Treatment | Relaxation + Cognitive restructuring + BoNT | NA | Overall Symptoms (subjective) | Improvement in subjective motor symptom | IV | |
| N | 1 | Details | Progressive muscle relaxation + Restructure cognition Timing of BoNT not mentioned | |||||||
| M/F | F | Frequency/Duration | Supervised: 1 session/w, 10w + Home: daily, 1y | |||||||
| Age (y) | Late 40 | |||||||||
| Silverman et al. [32] USA | RCT | Type | ADSD | Treatment | Voice training + BoNT | Sham voice training + BoNT & BoNT alone | BoNT effect, voice related QoL (BoNT effect, subjective) | All three groups significantly improved voice-related QoL No significant difference between groups | II | |
| N | 10/10/11 | Details | Voice education, relaxation, laryngeal massage, vocal exercises Voice training initiated 3w after BoNT | |||||||
| M/F | 5/26 | Frequency /Duration | 5 sessions, 12w | |||||||
| Age (y) | 48 (23–78) | |||||||||
| Murry et al. [33] USA | Case-control | Type | ADSD | Healthy control | Treatment | Voice therapy + BoNT | BoNT | Air flow rate (function) | Significant improvement in air flow rate Significant prolongation of interval between BoNT injections | III |
| N | 17 | 10 | Details | Voice therapy Voice training initiated within 3w after BoNT | ||||||
| M/F | 3/14 | 1/9 | Frequency/Duration | BoNT: 1 session + Voice therapy: 5 sessions, 9–54 w | ||||||
| Age (y) | 51 (27–74) | 52 (31–71) | ||||||||
| Focal Limb Dystonia | ||||||||||
| de Oliveira et al. [28] Brazil | Pre-post | Type | Writer’s cramp | Treatment | Progressive exercises + rTMS | NA | TWSTRS, WCRS (severity, pain) | Improvement in dystonia severity & pain | IV | |
| N | 1 | Details | Exercises for wrists and finger extensor muscles + rTMS (1Hz, 1200 pulses, 80%RMT, premotor cortex) | |||||||
| M/F | F | |||||||||
| Age (y) | 46 | Frequency/Duration | 15 sessions, 3 m | |||||||
| Kimberley et al. [43] USA | RCT cross-over | Type | Writer’s cramp | Treatment | Sensorimotor rehab + rTMS | Stretching & massage + rTMS | Global rating, arm dystonia disability scale (subjective, severity) | Both groups significantly improved subjective symptoms & dystonia severity. No significant difference between groups | II | |
| N | 9 | Details | Learning based sensorimotor rehab + rTMS (1Hz, 80% RMT, 1200 pulses, premotor cortex) | |||||||
| M/F | 6/3 | Frequency/Duration | 5d | |||||||
| Age (y) | 46 ± 10 | |||||||||
| Rosset-Llobet et al. [34] Spain | RCT | Type | Musician’s dystonia | Treatment | Sensorimotor rehab + tDCS | Sensorimotor rehab + sham tDCS | Dystonia severity score (severity) | Both groups significantly improved dystonia severity Adding tDCS showed significantly greater improvement | I | |
| N | 30 | Details | Splints to fingers with compensatory movements + tDCS (2 mA to parietal cortex) | |||||||
| M/F | 23/7 | Frequency/Duration | 1 h/session, 10 sessions, 2w | |||||||
| Age (y) | 35 ± 8 | |||||||||
| Furuya. et al. [35] Germany | Pre-post | Type | Musician’s dystonia | Treatment | Behavioral training + tDCS | NA | Key stroke (performance) | Significant improvement in music performance | III | |
| N | 10 | Details | Re-training of piano under regular tempo during tDCS (primary motor cortex) | |||||||
| M/F | 6/4 | |||||||||
| Age (y) | 24–61 | Frequency/Duration | 24 min/session, 5 sessions | |||||||
| Buttkus et al. [36] Germany | RCT cross-over | Type | Musician’s dystonia | Treatment | Sensorimotor rehab + tDCS | Sensorimotor rehab + sham tDCS | MIDI-based scale (severity) | No significant improvement in all three conditions | II | |
| N | 9 | Details | Retraining on piano + anodal or cathodal tDCS (2 mA, M1) | |||||||
| M/F | 9/0 | Frequency/Duration | 20 min, single session | |||||||
| Age (y) | 44 ± 11 | |||||||||
| Buttkus et al. [37] Germany | Case report, cross-over | Type | Musician’s dystonia | Treatment | Slow down exercise + anodal or cathodal tDCS | Sham tDCS | MIDI-based scale (severity) | Dystonia severity improved in all three conditions cathodal tDCS showed greater improvement | IV | |
| N | 1 | Details | Retraining on piano + anodal or cathodal tDCS (2 mA, M1) | |||||||
| M/F | M | Frequency/Duration | 20 min/session, 5 sessions/condition, 3 conditions, 21 w | |||||||
| Age (y) | 43 | |||||||||
| Functional Dystonia | ||||||||||
| Giorgi et al. 2024 [39] Italy | Case report | N | 1 | Treatment | Multimodal care | NA | Overall Symptoms (subjective) | Symptoms abated | IV | |
| M/F | M | Details | Mézières-Bertelè method + Tai chi + EMG biofeedback | |||||||
| Age (y) | 24 | Frequency/Duration | 1–3 sessions/w, 3 m | |||||||
| Antelmi. et al. [42] Italy | Case series | N | 2 | Treatment | Multimodal care | NA | Overall Symptoms (subjective) | One patient responded to rehabilitation One patient responded to BoNT | IV | |
| M/F | 0/2 | Details | BoNT, psychological and physical rehabilitation | |||||||
| Age (y) | 40, 65 | Frequency/Duration | NA | |||||||
| Vizcarra et al. [38] USA | RCT | N | 7 | 7 | Treatment | BoNT + CBT | Placebo BoNT + CBT | Psychiatric movement disorders rating scale (severity) | Both groups significantly improved dystonia severity No significant difference between groups | II |
| M/F | 1/6 | 2/4 | Details | Personalized CBT | ||||||
| Age (y) | 44 ± 15 | 53 ± 8 | Frequency/Duration | BoNT: 1 session + CBT: 1 session/w for 0–12 w, 12 w | ||||||
| Lee et al. [41] Germany | Case report | N | 1 | Treatment | Multimodal care | NA | Overall Symptoms (subjective) | No improvement | IV | |
| M/F | NA | Details | Splinting of the hand, PT, acupuncture | |||||||
| Age (y) | 21 | Frequency/Duration | several times, several days | |||||||
| Majumdar et al. [40] UK | Case series | N | 4 | Treatment | Multimodal care | NA | Overall Symptoms (subjective) | 2/4 patients with fixed dystonia responded | IV | |
| M/F | 0/4 | Details | BoNT, tenotomy, intensive PT, psychotherapy | |||||||
| Age (y) | 15 (13–19) | Frequency/Duration | NA | |||||||
| Ziegler et al. [44] (Germany) | Case report | N | 1 | Treatment | Multimodal care | NA | Overall Symptoms (subjective) | Symptoms abated | IV | |
| M/F | F | Details | CBT + PT | |||||||
| Age (y) | 11 | Frequency/Duration | 1.5 y of inpatient & outpatient care | |||||||
Individual study data for combination multimodal therapy for dystonia.
Values are shown as mean (standard deviation or SD), if SD was not available in the article min - max were shown if possible. AAN class, American Academy of Neurology classification framework; number of patients, n; male, M; female, F; year, y; month, week, w; hour, h; minute, min; second, s; not applicable, NA; Randomized controlled trial, RCT; transcranial direct current stimulation, tDCS; Toronto Western spasmodic torticollis rating scale, TWSTRS; botulinum toxin injections, BoNT; Craniocervical dystonia questionnaire, CDQ4; Quality of Life, QOL; Physical therapy, PT; repetitive Transcranial Magnetic Stimulation, rTMS; resting motor threshold, RMT; primary motor cortex; PMC, Writer’s Cramp Rating Scale, WCRS; Physical therapy, PT; Range of Motion, ROM; 36-Item Short-Form Health Survey, SF-36; functional electrical stimulation, FES; Activities of Daily Living, ADL; Transcutaneous electrical nerve stimulation, TENS; sensory-motor retraining, SMR; musical instrument digital interface, MIDI; Occupational Therapy, OT; electromyography, EMG; adductor spasmodic dysphonia, ADSD; cognitive behavioral therapy, CBT.
Focal cervical dystonia
Several studies investigated the effectiveness of incorporating supervised or home-based PT alongside ongoing BoNT injections [17–27]. In all these studies, PT was initiated after the BoNT injection had been administered. In four studies, PT was initiated immediately following the BoNT injection [18, 20, 23, 24], while in five other studies a gap ranging from about one week to one month was observed between the BoNT administration and the start of PT [17, 25–27]. One study did not specify the time between the two interventions [19]. The PT programs included stretching exercises, range-of-motion exercises, isometric neck muscle exercises, and feedback-based learning to control pathological movement patterns. Regardless of whether PT was administered alongside BoNT or not, studies conducted so far have reported notable improvements in dystonia severity, subjective symptoms, range-of-motion, and pain [18–24]. Tassorelli et al compared the effects of exercise therapy plus BoNT injections to BoNT injection therapy only [23]. They reported that participants receiving the combination therapy had greater reduction in pain levels, improvements in disability and prolongation of BoNT effects. PT was well tolerated with no reports of dystonia worsening or emergence of bothersome side effects [23]. In contrast, Stankovic et al. examined the role of adding BoNT to PT and compared it with PT alone. The study found that both groups receiving PT experienced improvements in disease severity but adding BoNT to PT resulted in superior and longer-lasting benefits [25]. In a study by Hu et al., a structured rehabilitation program combining supervised PT session followed by a home-based exercise program (including stretching, range-of-motion, and isometric exercises) with BoNT resulted in a significant reduction in dystonia severity compared to patients who received BoNT injections alone. There were no adverse events or worsening of symptoms [20]. In a RCT conducted in UK involving 110 patients with CD (90% of patients receiving BoNT), Counsell et al. examined the effects of individual supervised and specialized PT program that was based on Bleton’s technique involving the strengthening of underactive muscles, relaxation of overactive muscles, and guidance on head posture and body positioning. The control group received only posture advice, relaxation techniques, and home-based exercises for core stability and neck mobility. After six months, both PT groups demonstrated improvement on TWSTRS, but there was no significant difference between the groups. In both groups similar numbers of patients (3%–8%) reported subjective worsening of symptoms [22]. In another RCT conducted in Netherlands involving 96 CD patients and lasting one year, Van den Dool et al. compared a supervised and specialized PT program (customized to individual CD presentations also following Bleton’s technique) vs. supervised standard PT program both in combination with BoNT. They found that both PT programs resulted in similar improvements in patient-reported Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) disability. While no participants experienced worsening in the specialized PT program, 5 patients in the standard PT program reported subjective worsening [27]. Overall, there is evidence that PT in combination with BoNT serves as an effective therapy for CD.
TENS therapy commonly used for musculoskeletal conditions is a non-invasive treatment that uses low-voltage electrical currents to stimulate nerves and reduce pain. El-Bahrawy et al. compared the effects of a PT program (stretching exercises and voluntary movement training) plus TENS therapy plus BoNT vs. sham TENS plus BoNT [26]. They found that a combination of PT and TENS therapy for six weeks, when added to BoNT led to significant improvements of head posture and motor function. Treatments were tolerated well with no side effects reported.
Kinesiotaping is a therapeutic technique that involves applying elastic tape to the skin to provide support, improve proprioception, and modulate muscle activity via a combination of tension applied along the tape and stretching of the target muscle. That, amongst others, results in a change of recruitment activity patterns of the muscles and alleviates prolonged muscle contraction and even postural deviation [49]. Dec-Ćwiek M. et al. compared the effects of kinesiotaping plus BoNT injections vs. sham taping plus BoNT vs. BoNT alone. The study found that kinesiotaping (tape was applied to the shoulder muscles), when combined with BoNT, significantly improved QoL as measured by the Craniocervical Dystonia Questionnaire (CDQ)-24, however, there was no impact on dystonia severity. No side effects or worsening of symptoms were reported, except for one subject who experienced a skin rash after the first application of the tape [17]. Another study noted that a combination of kinesiotaping plus tDCS was effective in reducing dystonia severity and pain [28]. As some researchers are concerned about a placebo effect with the use of kinesiotaping, large RCTs are needed to establish their role.
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique to modulate cortical excitability. Bleton et al. examined the role of combining motor training with tDCS therapy and found greater and longer improvements in dystonia severity compared to tDCS alone [16]. Other combinations that showed clinical improvements included progressive exercises plus tDCS [34], and motor training plus repetitive TMS (rTMS) [29].
Biofeedback therapy is a technique that uses real-time monitoring of physiological signals (e.g., muscle activity, movement patterns) to help patients gain voluntary control over abnormal muscle activity. Gildenberg et al reported the effectiveness of biofeedback training plus TENS led to only partial improvements [30].
Meige syndrome and laryngeal dystonia
In a single case report, a combination of relaxation training and cognitive restructuring for 1 year in conjunction with BoNT therapy was found to improve symptoms of Meige syndrome [31]. Silverman et al. compared the effects of BoNT plus voice training vs. BoNT plus sham training vs. BoNT alone in patients diagnosed with adductor spasmodic dysphonia (ADSD) [32]. All three groups reported improvements in QoL without any side effects. However, there were no additional benefits to the use of voice training. In another study, Murry et al. found that voice training added to BoNT therapy in 17 ADSD patients led to greater improvements in airflow rate and significant prolongation of interval between BoNT injections when compared to BoNT alone [33].
Focal limb dystonia
Focal limb dystonia, such as hand dystonia, arises from an imbalance between agonist and antagonist muscle inhibition, leading to difficulties in executing fine motor tasks. This disruption is especially evident in individuals who perform highly repetitive, skill-based activities such as writing, playing musical instruments, or engaging in certain sports. The use of goal-directed and repeated sensory and motor behaviors can promote neuroplastic changes and potentially improve motor control and function. Rosset-Llobet et al. compared the effects of combining sensorimotor rehabilitation with tDCS (active vs. sham) in patients with MD. In their study, 26/28 participants completed the study, and no adverse events were noted. The study found improvements in dystonia severity in both groups receiving sensorimotor rehabilitation with further benefits when tDCS was added [34]. In another study, tDCS at the time of instrument retraining was reported to be effective in improving performance [35]. However, such benefits of combining training programs with neuromodulation was not consistently demonstrated. Buttkus et al. found the combination of sensorimotor rehabilitation and tDCS to be promising [36]. However, in their RCT, no clinical improvements were observed in MD when sensorimotor rehabilitation was combined with a single session of tDCS [36, 37]. Participants in the RCT complained of transient worsening fine motor control [36, 37]. Similarly, Kimberley et al. did not find the writing task to improve in patients with WC when sensorimotor training was combined with rTMS delivered to the premotor cortex. There was no worsening of dystonia or new adverse events noted [50].
Functional dystonia
In a study involving 14 patients with functional dystonia, including facial dystonia, CD, focal limb dystonia, and generalized dystonia; Vizcarra et al. investigated the effects of CBT with or without BoNT in managing dystonia symptoms. Both groups receiving CBT for 12 weeks reported significant improvements in dystonia symptoms, but the use of BoNT did not result in additional benefits beyond those observed with CBT alone. This suggests that CBT alone may be effective in managing symptoms of functional dystonia, regardless of the adjunctive use of BoNT [38]. Giorgi et al. reported that in a case of functional dystonia, symptoms completely abated after multimodal care, which included the Mézières-Bertelè method, Tai Chi, and electromyography (EMG) biofeedback therapy [39]. Similarly, Ziegler. et al. reported that multimodal care consisting of CBT and PT improved subjective perception of symptoms [44]. Majumdar. et al. examined the role of combining psychotherapy with BoNT, tenotomy, and intensive PT, in 4 adolescent patients with fixed dystonia (consistent with complex regional pain syndrome). In their cohort, two patients improved with these interventions; however, two did not [40]. Similarly, Lee. et al. reported that multimodal care such as splinting of the hand, PT, and acupuncture did not result in clinical improvements [41]. Antelmi. et al. applied multimodal care combining BoNT with psychological rehabilitation, and with PT in two patients respectively, with one patient responding to the rehabilitation and one patient responding to the BoNT [42].
Recommendation
A number of combination therapies can potentially improve CD symptoms. There is data from two class I studies of moderately large size that found supervised PT, whether customized to meet individual needs or following a standard protocol (stretching, range-of-motion exercises, strengthening exercises, and biofeedback), in combination with BoNT could reduce dystonia severity. Several studies, mostly of lower quality (Class II), suggest that combining therapies, such as pairing kinesiotaping with BoNT or integrating TENS with stretching and voluntary movement training alongside BoNT, may lead to improvements in motor control. Additionally, a combination of massage, passive myofascial elongation, stretching, and biofeedback could be considered as they could prolong BoNT effects. EMG based biofeedback therapy, combined with relaxation techniques, could improve head posture. Although it seems that most studies found the combination of PT and BoNT as beneficial in CD, the optimal timing for initiating PT following BoNT injections remains uncertain, and this timing may influence therapeutic outcomes. Further research is necessary to establish the optimal timing to maximize patient benefits.
In LD, a single study indicated that voice education, relaxation, laryngeal massage, and vocal exercises could improve the voice quality [32]. While BoNT is regarded as the gold standard and the only available treatment for improving symptoms of LD, conventional voice and speech therapy protocols have not been found to be effective in clinical experience. A consensus panel recommended that a subset of LD patients that exhibit symptoms of muscle tension dysphonia (characterized by excessive vocal effort due to increased tension in laryngeal and extra-laryngeal muscles) may show improvement with behavioral and speech therapy [47]. More research is needed to explore interventions focused on patient education, counseling, and the development of effective speaking strategies, particularly to address the heightened anxiety many individuals experience in social and occupational communication settings [47].
In focal limb dystonia, a single high-quality (Class I) study suggests that combining sensorimotor rehabilitation with splinting of fingers that display compensatory movements, along with tDCS for central neuromodulation, shows promise for treating MD. However, the evidence for treating WC with sensorimotor rehabilitation with additional neuromodulation using rTMS instead of tDCS (and no splinting) remains of lower quality. While CBT should be employed for treating functional dystonia, more data is needed to examine the role of intensive psychotherapy, PT, and OT in large sample studies to determine if the benefits remain sustained.
Effects of exercise, stretching, relaxation, biofeedback
We identified 32 studies in this category, with individual study results presented in Table 2. Among these studies, 11 focused on CD, 18 on TSD (10 MD, 10 WC), one on LD, and two on functional dystonia. The study designs included four RCTs, one non-randomized crossover trial, two case-control studies, 15 pre-post studies, four case series, and seven case reports.
TABLE 2
| Exercise/Stretching/Relaxation/Biofeedback (n = 33) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Study design | Participant characteristics | Intervention | Assessment outcomes | Results | AAN class | ||||
| Active group | Control group | Active | Control | |||||||
| Focal Cervical Dystonia | ||||||||||
| Useros-Olmo et al. [51] Spain | Case-control | N | 15 | 12 | Treatment | Relaxation technique programs | Observation | SF-36, VAS, TWSTRS (subjective, pain, severity) | Significant improvements in all outcomes | III |
| M/F | 1/14 | 4/8 | Details | Aquatic Watsu therapy, autogenic training | ||||||
| Age (y) | 47 ± 14 | 54 ± 12 | Frequency/Duration | Supervised: 1 session/w + Home: 30min/day, 4w | ||||||
| Boyce et al. [52] Australia | RCT | N | 9 | 11 | Treatment | Semi-supervised active exercise program | Relaxation | TWSTRS, BDI (severity, depression) | Both groups significantly improved dystonia severity & depression. No significant difference between groups | II |
| M/F | 6/14 | Details | Exercise to correct dystonic head position, relaxation | |||||||
| Age (y) | 57 ± 7 | Frequency/Duration | Supervised: 30 min session/w, 8w + Home: daily, 12w | |||||||
| Zetterberg et al. [53] Sweden | Pre-post | N | 6 | Treatment | Supervised PT | NA | TWSTRS, CDQ24, pain scale (severity, pain, subjective) | Significant improvement in all outcomes | III | |
| M/F | 2/4 | Details | Progressive muscle relaxation, isometric muscle endurance, dynamic strength | |||||||
| Age (y) | 48 (30–59) | Frequency/Duration | 45 min/session, 36 session, 4 w | |||||||
| Smania et al. [54] Italy | Cross-over | N | 4 | Treatment | Supervised PT | Standard biofeedback program | Head realignment, questionnaire, VAS (function, subjective, pain) | Both groups significantly improved pain & head-trunk alignment. No significant difference between groups | III | |
| M/F | 1/3 | Details | Postural reeducation exercises, passive elongation of myofascial cervical structures | |||||||
| Age (y) | 41.7 | Frequency/Duration | 1 h/session, 5 d/w, 5 w | |||||||
| Duddy et al. [55] UK | RCT | N | 6 | 5 | Treatment | Biofeedback training | Television monitored relaxation only | EMG, speech evaluation (physiological study, subjective) | Both groups significantly improved subjective symptoms No significant difference between groups | II |
| M/F | 5/6 | Details | EMG biofeedback, television monitored relaxation | |||||||
| Age (y) | 33–66 | Frequency/Duration | 2 session/w, 4 w | |||||||
| Spencer et al. [56] USA | Case report | N | 1 | Treatment | Supervised PT | NA | Subjective, head posture, EMG (subjective, function, physiological study) | Improvement in ROM & head posture | IV | |
| M/F | M | Details | Strengthen antagonists, correction of head posture with mirror | |||||||
| Age (y) | 29 | Frequency/Duration | 7 sessions, 20 w | |||||||
| Leplow et al. [57] Germany | Pre-post | N | 10 | Treatment | Biofeedback training | NA | Subjective symptoms | Significant improvement | IV | |
| M/F | 6/4 | Details | EMG biofeedback, relaxation, stress-management, counselling | |||||||
| Age (y) | 42–61 | Frequency/Duration | 45 min/session, 14–25 sessions | |||||||
| Jahanshahi et al. [58] UK | RCT | N | 6 | 6 | Treatment | EMG biofeedback + relaxation | Relaxation | ROM, EMG (function, physiological study) | Both groups significantly improved in head position & neck mobility | II |
| M/F | 3/3 | 2/4 | Details | Learning to relax tense sternocleidomastoid | ||||||
| Age (y) | 48 (26–68) | 57 (48–60) | Frequency/Duration | 1–2 session/w, 27 sessions, 3 m | ||||||
| Cleeland et al. [59] USA | Pre-post | N | 10 | Treatment | Biofeedback training | NA | Overall Symptoms (subjective) | Improvement in 9/10 patients | IV | |
| M/F | 4/6 | Details | EMG biofeedback (auditory & electrical shock) | |||||||
| Age (y) | 15–64 | Frequency/Duration | 6–23 sessions | |||||||
| Martin et al. [60] UK | Case series | N | 6 | Treatment | Biofeedback training + Home training | NA | EMG, ROM (physiological study, function) | All patients improved to some extent | IV | |
| M/F | 0/6 | Details | Conventional 4 sessions + Specifically designed 4 sessions | |||||||
| Age (y) | 40–63 | Frequency/Duration | Biofeedback: 20 session/d, 3d + Home: daily, 1w | |||||||
| Brudny et al. [61] USA | Pre-post | N | 69 | Treatment | Biofeedback training | NA | Overall Symptoms (subjective) | Improvement in 37/69 patients | III | |
| M/F | NA | Details | EMG biofeedback | |||||||
| Age (y) | NA | Frequency/Duration | 3–5 sessions/w, 8-12w | |||||||
| Laryngeal Dystonia | ||||||||||
| Keatley et al. [62] UK | Case report | N | 1 | Treatment | Speech therapy | NA | Speech evaluation (subjective, performance) | Specifically designed showed greater improvement in subjective symptoms & speech performance | IV | |
| M/F | M | Details | Speech therapy specifically designed to reduce lip tension | |||||||
| Age (y) | 62 | Frequency/Duration | Conventional: 4 sessions + Specifically designed: 4 sessions | |||||||
| Focal Limb Dystonia | ||||||||||
| Ackermann et al. [63] Australia | Pre-post | Type | Musician’s dystonia | Treatment | Anatomy based retraining program | NA | Tubiana and Chamagne Scale (performance) | Improvement in music performance | IV | |
| N | 4 | Details | Progressive muscle activation, movement exercise program | |||||||
| M/F | 2/2 | Frequency/Duration | Every day, 12 m | |||||||
| Age (y) | 27.8 | |||||||||
| Butler et al. [64] UK | Pre-post | Type | Musician’s dystonia | Writer’s cramp | Treatment | Sensorimotor rehab | NA | Arm dystonia disability (severity) | Slight improvement in dystonia severity | III |
| N | 7 | 5 | Details | mirror therapy, slow down exercise, ultrasound, re-education, exercise, stretch | ||||||
| M/F | 4/8 | Frequency/Duration | Supervised: 30–60 min/session, 6 sessions + Home: daily, 6 m | |||||||
| Age (y) | 51 | |||||||||
| Yoshie et al. [65] Japan | Case report | Type | Musician’s dystonia | Treatment | Slow down exercise training | NA | Key stroke (performance) | Regularity of keystrokes improved | IV | |
| N | 1 | Details | Simple five-finger motor task | |||||||
| M/F | F | Frequency/Duration | 30 min/d, every day, 1y | |||||||
| Age (y) | 25 | |||||||||
| Hashimoto et al. [66] Japan | Case report | Type | Writer’s cramp | Treatment | Brain-computer interface rehabilitation | NA | Handwriting test (performance) | Improvement in writing performance | IV | |
| N | 1 | Details | Visual EEG feedback from sensorimotor cortex | |||||||
| M/F | F | |||||||||
| Age (y) | 67 | Frequency/Duration | 60 min/session, 10 sessions, 5 m | |||||||
| Cheng et al. [67] Germany | Case-control | Type | Musician’s dystonia | Healthy control | Treatment | Altered auditory feedback | No or delayed feedback | Key stroke (performance) | No significant improvement in both groups | III |
| N | 12 | 25 | Details | Input at tempo of 4 notes/min, 80 beats/min | ||||||
| M/F | 8/4 | 13/12 | Frequency/Duration | 1 min | ||||||
| Age (y) | 44 (9) | 25 (3) | ||||||||
| de Lisle et al. [68] New Zealand | Case report | Type | Musician’s dystonia | Treatment | Instrumental retraining | NA | Music performance scales (performance) | Improvement in music performance | IV | |
| N | 1 | Details | Upper limb reversing position | |||||||
| M/F | F | Frequency/Duration | 18 sessions, 5w | |||||||
| Age (y) | 42 | |||||||||
| Baur et al. [69] Germany | Pre-post | Type | Writer’s cramp | Treatment | Biofeedback training | NA | Writing frequency, fluency, pressure (performance) | Significant improvement in writing performance | III | |
| N | 7 | Details | Auditory grip force feedback training, writing strategies | |||||||
| M/F | 3/4 | Frequency/Duration | 60 min/session, 7 sessions, 2-7w | |||||||
| Age (y) | 52 (44–65) | |||||||||
| Byl et al. [70] USA | Pre-post | Type | Writer’s cramp | Treatment | Home-based sensorimotor training | NA | Task specific performance (performance) | Significant improvement in writing performance | III | |
| N | 13 | Details | Imagery of normal movement, learning to interface hand with target instrument, sensorimotor training | |||||||
| M/F | 10/3 | Frequency/Duration | 3 h/d, 5 d/w for 2w in Phase I; 5 h/d for 1w in Phase II; 8w | |||||||
| Age (y) | 47 (27–66) | |||||||||
| McKenzie et al. [71] USA | Pre-post | Type | Musician’s dystonia | Writer’s cramp | Treatment | Learning based sensorimotor training | NA | Physical, sensory, & motor performance | Both groups significantly improved task specific performance | III |
| N | 14 | 13 | Details | Education on healthy habits, home program | ||||||
| M/F | 7/7 | 4/9 | Frequency/Duration | 2 h/d home training, 8w | ||||||
| Age (y) | 42 ± 11 | 44 ± 10 | ||||||||
| Berger et al. [72] Netherlands | Pre-post | Type | Writer’s cramp | Treatment | Biofeedback training | NA | writing test | Significant improvement in handwriting | III | |
| N | 5 | Details | Muscle feedback recorded with EMG during writing | |||||||
| M/F | 5/0 | Frequency/Duration | 5–10 sessions, 5–14 m | |||||||
| Age (y) | 28–54 | |||||||||
| de Lisle [73] New Zealand | Case series | Type | Musician’s dystonia | Treatment | Instrumental retraining | NA | Music performance scales (performance) | Improvement in music performance | IV | |
| N | 3 | Details | Motor practice with proper body biomechanics | |||||||
| M/F | NA | Frequency/Duration | 10 sessions, 2 w | |||||||
| Age (y) | NA | |||||||||
| Sakai et al. [74] Japan | Case series | Type | Musician’s dystonia | Treatment | Slow-down exercise | NA | Music performance scales (performance) | Improvement in music performance | IV | |
| N | 20 | Details | Practice playing piano at slowed speed | |||||||
| M/F | 10/10 | Frequency/Duration | 30min/day, daily, 1–6 y | |||||||
| Age (y) | 30 | |||||||||
| Schenk et al. [75] UK | Pre-post | Type | Writer’s cramp | Treatment | Writing retraining | NA | Handwriting kinematics (performance) | Significant improvements | III | |
| N | 50 | Details | Supervised motor exercises | |||||||
| M/F | 21/29 | Frequency/Duration | 50–60 min/session, 1 session/1-4w, 2–20 sessions, 4 m | |||||||
| Age (y) | 44 ± 11 | |||||||||
| Byl et al. [76] USA | Pre-post | Type | Musician’s dystonia | Treatment | Sensory discrimination training | NA | Task specific performance (performance) | Significant improvement | III | |
| N | 3 | Details | Sensorimotor training, stress-free use for hand, aerobics, postural exercises | |||||||
| M/F | 1/2 | Frequency/Duration | Supervised: 19–23 sessions + Home: daily, 12 w | |||||||
| Age (y) | 23,35,24 | |||||||||
| Zeuner et al. [77] USA | Pre-post | Type | Writer’s cramp | Treatment | Sensory training | NA | Fahn dystonia scale (severity) | Significant improvement in dystonia severity | III | |
| N | 10 | Details | Braille reading at grade 1 level | |||||||
| M/F | 1/9 | Frequency/Duration | 30–60 min/d, daily, 8 w | |||||||
| Age (y) | 50 ± 7 | |||||||||
| Byl et al. [78] USA | Case series | Type | Musician’s dystonia | Treatment | Sensory discriminative training + Home program | NA | Sensory Integration and Praxis Test; strength; ROM, VAS (severity, function, pain) | Significant improvements in all outcomes | IV | |
| N | 12 | Details | Posture, relaxation, mobilization, fitness, motor imagery | |||||||
| M/F | NA | Frequency/Duration | 6–18 w | |||||||
| Age (y) | NA | |||||||||
| Deepak et al. [79] India | Pre-post | Type | Writer’s cramp | Treatment | Biofeedback training | NA | VAS, EMG (performance, pain, physiological study) | Writing performance and pain improved in 9/10 patients | IV | |
| N | 10 | Details | Audio-feedback EMG from abnormally activity muscles during writing practice | |||||||
| M/F | 10/0 | Frequency/Duration | 4 sessions minimum, 8 w | |||||||
| Age (y) | 19–62 | |||||||||
| O'Neill. et al. [80] (USA) | Case report | Type | Writer’s cramp | Treatment | Biofeedback training | NA | Self-report, EMG (subjective, physiological study) | Subjective improvement & decrease in EMG amplitude | IV | |
| N | 1 | Details | EMG biofeedback (visual + auditory) during handwriting practice | |||||||
| M/F | M | Frequency/Duration | Supervised: 2 sessions + Home: daily, 2w | |||||||
| Age (y) | 52 | |||||||||
| Functional Dystonia | ||||||||||
| Gros et al. 2024 [81] Canada | Pre-post | N | 4 | Treatment | PT | NA | Subjective symptoms (subjective) | All patients reported subjective improvement | IV | |
| M/F | 1/3 | Details | Symptom-based individualized rehabilitation | |||||||
| Age (y) | 22–32 | Frequency/Duration | 3m - 1y | |||||||
| Stephen. et al. [82] USA | Case report | N | 1 | Treatment | PT | NA | Subjective symptoms (subjective) | No significant improvement | IV | |
| M/F | F | Details | NA | |||||||
| Age (y) | 41 | Frequency/Duration | NA | |||||||
Exercise/Stretching/Relaxation/Biofeedback therapy for dystonia.
Values are shown as mean (standard deviation or SD), if SD was not available in the article min - max were shown if possible. AAN class, American Academy of Neurology classification framework; number of patients, n; male, M; female, F; year, y; month, week, w; hour, h; minute, min; second, s; not applicable, NA; Randomized controlled trial, RCT; 36-Item Short-Form Health Survey, SF-36; Visual Analogue Scale, VAS; Toronto Western spasmodic torticollis rating scale, TWSTRS; Physical therapy, PT; Beck Depression Inventory, BDI; Cervical Dystonia Questionnaire, CDQ; writer’s cramp rating scale, WCRS; single-photon emission computed tomography, SPECT; The Burke–Fahn–Marsden Dystonia Rating Scale, BFMDRS; Electromyography. EMG; Range of Motion, ROM; electroencephalogram, EEG.
Focal cervical and laryngeal dystonia
Multiple small studies have demonstrated the effectiveness of individually supervised PT training in improving dystonia severity, range-of-motion, and head posture however the sample sizes have been small with the quality of evidence deemed low or very low [53, 56]. In one RCT, Boyce MJ et al. found that in CD, combining active exercises aimed at correcting dystonic head position and relaxation therapy for neck muscles was not more effective than relaxation therapy alone. While there were no reports of severe side effects, exercises resulted in mild muscle soreness [52]. Biofeedback training can also be effective to improve symptoms in CD. In an RCT, Duddy. et al. compared the EMG-based biofeedback to relaxation training, to find similar improvements in the two groups. There were no side effects or worsening of symptoms [55]. Smania N et al. found that whether patients received posture education with passive elongation of myofascial cervical structures or received standard biofeedback therapy, there were similar improvements in posture and pain [54]. While relaxation techniques such as Aquatic Watsu therapy and autogenic training were found to improve subjective symptoms, pain, and dystonia severity [51], a few small-scale studies may support the effectiveness of biofeedback alone, however the quality of evidence is low or very low [57, 59–61]. Jahanshahi M et al. examined the role of EMG-based biofeedback when added to relaxation training vs. relaxation training alone. Both groups reported clinical improvements in dystonic head position and neck mobility [58].
Regarding LD, we found only a single case report of speech therapy program implemented in a patient with long standing dystonia that was specifically designed to reduce lip tension. After eight weekly sessions, the patient was reported to subjectively improve and speech production to a greater degree than conventional speech therapy [62].
Focal limb dystonia
Byl et al. reported that sensory discrimination training and sensorimotor training in patients with WC and MD even when applied as home-based therapy can potentially improve task-specific performance [70, 76, 78]. Similarly, McKenzie. et al. found learning-based sensorimotor training to improve task-specific performance in both WC and MD groups [71]. Sensorimotor rehabilitation was found to improve symptoms in many other case reports and small-scale studies. However, the quality of evidence was low to very low [63–65, 68, 73–75, 77]. Some studies found that sensorimotor training involving EMG-based biofeedback was effective in improving writing performance in WC [72]. Biofeedback therapy was ineffective in MD, but it improved writing performance and pain in WC [66, 67, 69, 79, 80].
Functional dystonia
Gros et al. examined the role of symptom-based individualized rehab employed for 3 months to 1 year in four patients diagnosed with functional dystonia (fixed hand dystonia, lower limb dystonia, episodic facial dystonia, axial dystonia). They found that through education, desensitization, promotion of normal movement pattern, relaxation, and psychotherapy, all patients reported subjective improvements [81]. Another case report found that PT, offered at a specialized dystonia clinic, was beneficial in a patient with functional dystonia presenting with fixed dystonia of the hand and lower limb, accompanied by nearly continuous, high-amplitude, irregular tremor and pain in the head and limbs [82].
Recommendation
There is low quality evidence for biofeedback training and relaxation techniques like Watsu or autogenic training that patients may experience improvements. Similarly, studies in focal hand dystonia, though limited by low-quality evidence, have demonstrated benefits from sensorimotor retraining and biofeedback therapy. There is minimal data in LD as only a single case report was found describing the use of speech therapy in a patient with a longstanding history of dysarthria. Finally, PT for functional dystonia may be effective, though further studies are needed to confirm their benefits.
Role of employing externally applied modalities (kinesiotaping, vibrotactile stimulation, TENS and use of adaptive splints and orthotic device)
We identified 22 studies in this category, including two in CD, one in LD, 11 in WC, seven in MD, and one in focal leg dystonia. Individual study results are detailed in Table 3. We identified four studies as RCTs, including trials with a cross-over design. Additionally, two were non-randomized cross-over trials, one was a case-control study, 12 utilized a pre-post design, and the remaining were case reports.
TABLE 3
| Adaptive aids or (kinesiotapes, splints, orthotic devices, vibrotactile stimulators, TENs) (n = 24) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Study design | Participant characteristics | Intervention | Assessment outcomes | Results | AAN class | ||||
| Active group | Control group | Active | Control | |||||||
| Focal Cervical Dystonia | ||||||||||
| Xu et al. [83] USA | Pre-post | N | 44 | Treatment | Vibrotactile stimulation | NA | Perceived pain score (0–100 pain scale) | Significant improvement in pain | III | |
| M/F | 15/29 | Details | Small electric vibratory motors stimulated sternocleidomastoid ± trapezius muscles | |||||||
| Age (y) | 61.8 | Frequency/Duration | 45 min stimulation, single session | |||||||
| Pelosin et al. [84] Italy | RCT cross-over | N | 12 | Treatment | Kinesiotaping | Sham taping | VAS, TWSTRS (pain, severity) | Significant improvement in pain No significant improvement in dystonia severity | II | |
| M/F | 4/8 | Details | Kinesiotape applied to skin over affected muscles | |||||||
| Age (y) | 55 ± 9 | Frequency/Duration | Taping every day, 2 w/condition, 1m/washout, 2 m | |||||||
| Laryngeal dystonia | ||||||||||
| Khosravani et al. [85] USA | Pre-post | N | 13 | Type | Vibrotactile stimulation | NA | Voice analysis (performance) | 9 participants had less voice breaks & better voice quality | IV | |
| M/F | 5/8 | Details | vibro-motors attached to skin over thyroid cartilage were used during vocalization for laryngeal vibration | |||||||
| Age (y) | 58 ± 12 | Frequency/Duration | Single session for ∼30min | |||||||
| Focal limb dystonia | ||||||||||
| Bravi et al. [86] Italy | Cross-over | Type | Musician’s dystonia | Treatment | Kinesiotaping | Sham Kinesiotaping | VAS (performance) | No significant improvement in music performance | III | |
| N | 7 | Details | Kinesiotape applied to skin over affected dystonic fingers as well as compensatory fingers | |||||||
| M/F | NA | |||||||||
| Age (y) | 35 ± 9 | Frequency/Duration | Single session lasting ∼8 min | |||||||
| Oborzyński et al. [87] Poland | Pre-post | Type | Writer’s cramp | Treatment | Immobilization with splints | NA | BFMDRS, Arm Dystonia Disability Scale (severity) | No significant improvement | III | |
| N | 9 | Details | Thermoplastic splint designed for each individual’s dystonia was applied to immobilize dystonic fingers and wrist | |||||||
| M/F | 1/8 | Frequency/Duration | 1 h/d, 5 d/w, 3 w | |||||||
| Age (y) | 44.3 ± 10.6 | |||||||||
| Barrett et al. [88] Netherlands | Case report | Type | Lower limb dystonia | Treatment | Functional electrical stimulation of peroneal nerve | NA | 6-min walk test, single leg stance time, TUG time (performance) | Improvement in 6-min walk & single leg stance time Worsened TUG time | IV | |
| N | 1 | Details | Fitted with radio frequency-controlled device that patient used on daily basis | |||||||
| M/F | F | |||||||||
| Age (y) | 62 | Frequency/Duration | Daily, 18 months | |||||||
| Singam et al. [89] USA | Pre-post | Type | Writer’s cramp | Treatment | Sensorimotor retuning with writing orthotic device | NA | WCRS, VAS (performance) | Improvement in writing performance | III | |
| N | 11 | Details | Modifying standard handwriting posture with plastic orthotic device | |||||||
| M/F | NA | |||||||||
| Age (y) | 53 ± 12 | Frequency/Duration | Daily home practice, 2 w | |||||||
| Pelosin et al. [84] Italy | RCT cross-over | Type | Writer’s cramp | Treatment | Kinesiotaping | Sham taping | VAS, WCRS (pain, severity) | Significant decrease in pain in both groups but no change in dystonia severity | II | |
| N | 10 | Details | Kinesiotape applied to skin over the affected muscle | |||||||
| M/F | 3/7 | |||||||||
| Age (y) | 48 ± 6 | Frequency/Duration | Taping every day, 2 w/condition, 1m/washout, 2 m | |||||||
| Waissman et al. [90] Brazil | Pre-post | Type | Writer’s cramp | Treatment | Immobilization of dystonic fingers with splint | NA | BFMDRS, Analog Pain Scale (severity, pain) | Significant improvement in pain & dystonia severity | III | |
| N | 12 | Details | Writing training followed by finger immobilization with specific ring in eight splints | |||||||
| M/F | 7/5 | |||||||||
| Age (y) | 52 ± 16 | Frequency/Duration | Supervised: 60 min/d, 2 d/w, 8 w + Home: daily, 8 w | |||||||
| Berque et al. [91] UK | Pre-post | Type | Musician’s dystonia | Treatment | Immobilization of non-dystonic compensatory finger movements with splint | NA | Frequency of abnormal movement (severity) | Significant improvement in dystonia severity | IV | |
| N | 4 | Details | Retraining of motor control at slow speed + immobilization with splint | |||||||
| M/F | 7/1 | Frequency/Duration | Supervised: 2 h/d, 1 w + Home: 30–60 min/day, 4 y | |||||||
| Age (y) | 48 (30–55) | |||||||||
| Rosset-Llobet et al. [92] Spain | Case report | Type | Musician’s dystonia | Treatment | Sensorimotor retuning: Immobilization of non-dystonic compensatory finger movements with splint | NA | Subjective assessment (subjective) | Improvement in subjective symptoms | IV | |
| N | 1 | Details | Splinting of non-dystonic fingers and exercises for dystonic fingers | |||||||
| M/F | M | |||||||||
| Age (y) | 45 | Frequency/Duration | Daily practice, 1 y | |||||||
| Meunier et al. [93] France | RCT cross-over | Type | Primary writing tremor | Treatment | TENS applied to wrist flexor muscles | Sham stimulation | Fahn–Tolosa–Marin Tremor Rating Scale (severity) | TENS at 5 and 25 Hz did not have any effect while TENS at 50 Hz worsened the clinical condition | II | |
| N | 9 | Details | 120% RMT, 250 μs, 5, 25 or 50 Hz, in 2-s trains separated by 2-s pauses | |||||||
| M/F | 9/0 | |||||||||
| Age (y) | 62 ± 13 | Frequency/Duration | 14 sessions (7 days for per week for two consecutive weeks) lasting 20 min each | |||||||
| Waissman et al. [94] Brazil | Pre-post | Type | Writer’s cramp | Treatment | Immobilization of dystonic fingers with splint | NA | BFMDRS, Jedynak Writing Evaluation, pain scale (severity, performance, pain) | Improvements in all measures | III | |
| N | 2 | Details | Writing training followed by finger immobilization with specific splints | |||||||
| M/F | 1/1 | |||||||||
| Age (y) | 24, 44 | Frequency/Duration | Supervised: 60 min/d, 2 d/w, 8 w + Home: daily, 8 w | |||||||
| Berque et al. [95] UK | Pre-post | Type | Musician’s dystonia | Treatment | Immobilization of non-dystonic compensatory finger movements with splint | NA | Frequency of abnormal movement (severity) | Significant improvement in dystonia severity | IV | |
| N | 8 | Details | Retraining of motor control at slow speed + immobilization with splint | |||||||
| M/F | 7/1 | Frequency/Duration | Supervised: 2 h/d, 1 w + Home: 30–60 min/day, 1 y | |||||||
| Age (y) | 48 (30–55) | |||||||||
| Trompetto et al. [96] Italy | Cross-over | Type | Writer’s cramp | Treatment | Extracorporeal shockwave therapy | Placebo shock | UDRS, Arm dystonia disability scale | Improvement in dystonia severity | III | |
| N | 3 | Details | 800–3000 pulses to dystonic muscles in hand | |||||||
| M/F | 0/3 | |||||||||
| Age (y) | 41,47,25 | Frequency/Duration | 1 session/w, 4 w | |||||||
| Zeuner et al. [97] Germany | RCT | Type | Writer’s cramp | Treatment | Immobilization of non-dystonic compensatory finger movements followed by retraining | task non-specific motor re-training, use of therapeutic putty | WCRS (severity, performance) | Both groups significantly improved in dystonia severity & writing performance (pre-post) | I | |
| N | 26 | Details | Drawing & writing exercises with stax finger splints (pen attached) | |||||||
| M/F | 14/12 | |||||||||
| Age (y) | 49 ± 12 | Frequency/Duration | 35–60 min/d, daily, 8 w | |||||||
| Tinazzi et al. [98] Italy | Case-control | Type | Writer’s cramp | Healthy control | Treatment | TENS over forearm agonist & antagonist muscles | NA | Writing performance | Significant improvement in writing time | III |
| N | 10 | 14 | Details | 1.5 mA below motor threshold, 2s trains at 150 Hz | ||||||
| M/F | 8/6 | NA | ||||||||
| Age (y) | 33 | NA | Frequency/Duration | 30 min/session, 5 sessions/w, 3w | ||||||
| Tinazzi. et al. [99] Italy | RCT cross-over | Type | Writer’s cramp | Treatment | TENS over forearm flexor muscles | Sham ultrasound | Dystonia movement scale, writing test, VAS (severity, subjective, performance) | Significant improvement in all outcomes | II | |
| N | 10 | Details | 50 Hz, 250 μs, below pain threshold, 2s trains | |||||||
| M/F | 5/5 | |||||||||
| Age (y) | 33 ± 4 | Frequency/Duration | 20 min/session, 5 sessions/w, 2w | |||||||
| Zeuner et al. [100] USA | Pre-post | Type | Writer’s cramp | Treatment | Immobilization of non-dystonic compensatory finger/wrist and motor training of affected fingers | NA | BFMDRS, kinematic analysis of handwriting (severity, performance) | Mild subjective improvement | III | |
| N | 10 | Details | Train each finger individually with splint & pen | |||||||
| M/F | NA | |||||||||
| Age (y) | 54.0 ± 8.4 | Frequency/Duration | 25 min/d for 1w + 50 min/d for 3-7w | |||||||
| Pesenti et al. [101] Italy | Case series | Type | Musician’s dystonia | Writer’s cramp | Treatment | Dystonic hand immobilization | NA | Subjective and performance scale, hand grip test (subjective, performance, function) | Variable outcomes at follow-up | IV |
| N | 15 | 4 | Details | Immobilization with plastic splint applied to fingers and wrist | ||||||
| M/F | NA | |||||||||
| Age (y) | NA | Frequency/Duration | Daily, 4–5 w | |||||||
| Candia. et al. [102] Germany | Pre-post | Type | Musician’s dystonia, Embouchure dystonia | Treatment | Sensory motor retuning | NA | Dystonia evaluation scale, music performance test (severity, performance) | Pianists & guitarists showed improvement Not embouchure dystonia | IV | |
| N | 11 | Details | Immobilizes one or more compensatory finger(s) with splint; Repetitive exercises for dystonic finger | |||||||
| M/F | 8/3 | |||||||||
| Age (y) | 40 (30–70) | Frequency/Duration | Supervised: 1.5–2.5 h/day, 8d + Home: 1 h, daily, 1y | |||||||
| Priori et al. [103] Italy | Pre-post | Type | Musician’s dystonia | Treatment | Immobilization therapy with splint | NA | Arm dystonia disability scale, Tubiana Chamagne score (severity, subjective, performance) | Significant improvement in dystonia severity & music performance (pianists, guitarists, drummers) | IV | |
| N | 8 | Details | Finger & wrist joints of dystonic hand immobilized with plastic splint | |||||||
| M/F | 7/1 | |||||||||
| Age (y) | 30 ± 6 | Frequency/Duration | 4.5 ± 0.75 w | |||||||
| Candia. et al. [104] Germany | Pre-post | Type | Musician’s dystonia | Treatment | Sensory motor retuning | NA | Dystonia evaluation scale (severity) | Significant improvement in dystonia severity | IV | |
| N | 5 | Details | Immobilization by splint(s) of one or more compensatory digits other than focal dystonic finger. Repetitive exercises for dystonic finger | |||||||
| M/F | NA | |||||||||
| Age (y) | NA | Frequency/Duration | 1.5–2.5 h/d, 8d | |||||||
| Focal limb dystonia | ||||||||||
| Ferrara et al. [105] | Pre-post | N | 12 | Treatment | TENS over muscles that were maximally affected | NA | Psychogenic Movement Disorders Rating Scale (severity) | 5/12 showed significant improvement in dystonia severity | IV | |
| M/F | NA | Details | Stimulus strength was titrated to produce a tingling sensation in the stimulated area without muscle twitching or pain, 2-s trains, 150 Hz | |||||||
| Age (y) | NA | Frequency/Duration | 30 min/day, daily, 6.9 ± 4.7 m | |||||||
Adaptive Aids or Devices (Kinesiotape, Splints, Vibrotactile stimulation, Orthotic device, TENS and FES).
Values are shown as mean (standard deviation or SD), if SD was not available in the article min - max were shown if possible. AAN class, American Academy of Neurology classification framework; number of patients, n; male, M; female, F; year, y; month, week, w; hour, h; minute, min; second, s; not applicable, NA; Randomized controlled trial, RCT; Visual Analogue Scale, VAS; Toronto Western spasmodic torticollis rating scale, TWSTRS; Writer’s cramp rating scale, WCRS; electroencephalography, EEG; functional electrical stimulation, FES; timed up and go test, TUG; The Burke–Fahn–Marsden Dystonia Rating Scale, BFMDRS; the Unified Dystonia Rating Scale, UDRS; nerve conduction study, NCS; Somatosensory Evoked Potential test, SEP; Radial extracorporeal shockwave therapy, rESWT; Transcutaneous electrical nerve stimulation, TENS; Motor evoked potentials, MEP; Physical therapy, PT; resting motor threshold, RMT.
Focal cervical and laryngeal dystonia
Pelosin et al. compared the effects of 2 weeks of kinesiotaping to sham taping in 12 patients with CD. Kinesiotaping applied to the shoulder and neck muscles was found to significantly reduce subjective pain sensation, however, it had no effects on the severity of symptoms. No side effects or symptom worsening was found after intervention [84]. Xu et al. found that a single session of vibrotactile stimulation applied to neck muscles in 44 CD patients was able to significantly reduce subjective pain perception [83]. However, vibrotactile stimulation, when applied to laryngeal area (lateral parts of thyroid cartilage) in patients with LD did not significantly reduce voice breaks and improve voice quality [85].
Focal limb dystonia
Bravi et al. compared the effects of kinesiotaping vs. sham taping in MD but did not find significant improvements [86]. Similarly, in a crossover design study, Pelosin et al. evaluated kinesiotaping in patients with WC and found no significant benefits, as pain improvements were comparable to those observed with sham taping [84]. Many studies have investigated the role of splints in improving TSD symptoms. These devices were used either to immobilize dystonic fingers while allowing the use of unaffected fingers or to immobilize the unaffected fingers to control overflow compensatory movements and enable motor training for the affected fingers. Zeuner et al. conducted two studies on WC where they combined the use of splints with motor training lasting about 7–8 weeks. In both studies, the use of splints was found to improve the writing performance [97, 100]. In their experience, dystonia transiently worsened immediately after immobilization in some patients however returned to baseline levels during subsequent training [97]. Berque et al, Candia et al. and Priori et al. evaluated the effectiveness of splints in patients with MD to find improvements in dystonia severity and musical performance (pianist, guitarist or drummer) [87, 90–92, 94, 95, 101–104, 106]. Regardless of whether the splints were applied to dystonic or non-dystonic fingers, they were generally classified as low or very low quality, with only low or modest effect sizes reported. Another limitation of using finger splints is that they may be ineffective if dystonia involves multiple joints, especially when proximal movements contribute to the condition.
Other research groups examined the role of orthotic device and TENS therapy for improving symptoms of WC. Singam et al. reported that the use of a portable orthotic device to improve hand posture for 2 weeks led to an improvement of writing performance in WC [89]. Tinazzi et al. found that TENS therapy to forearm muscles in patients with WC led to a significantly shorter writing time [98, 99]. However Meunier et al in a cross-over, double-blinded randomized study found TENS therapy at certain frequencies were harmful in patients with primary writing tremor [93]. Other small studies presented the effectiveness of functional electrical stimulation for walking performance in lower extremity dystonia, and extracorporeal shock wave therapy for writing performance in patients with WC [88, 96].
Functional dystonia
In a study on functional movement disorders, 12 of 19 patients were found to have functional dystonia. Most participants experienced immediate benefits during their clinic visits. A total of 15 patients (79%) chose to continue using TENS therapy as outpatients, and five patients showed a significant (50% or greater) improvement in their Psychogenic Movement Disorders Rating Scale (PMDRS) scores. Daily 30-min TENS sessions were associated with improvements that were maintained at a six-month follow-up [105].
Recommendation
While kinesiotaping applied to the shoulder and neck muscles can reduce subjective pain sensation in CD, vibrotactile stimulation requires further investigation in both CD and LD. Kinesiotaping, TENS over the forearm muscles, and immobilization therapy with splints are promising in treating WC symptoms and MD, but high-quality evidence supporting their efficacy is lacking. It is also unclear at this point whether immobilization of affected dystonic fingers or unaffected fingers is more beneficial.
Role of behavioral interventions and psychotherapy
We identified eight studies in this category, with individual study results presented in Table 4. There were three studies on focal limb dystonia, including one RCT on WC, one case series, and one case report on sports-related TSD. Additionally, five case reports focused on functional dystonia.
TABLE 4
| Behavioral therapy (n = 8) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Study design | Participant characteristics | Intervention | Assessment outcomes | Results | AAN class | ||||
| Active group | Control group | Active | Control | |||||||
| Focal limb dystonia | ||||||||||
| Tibben et al. [107] Netherlands | Case series | Type | Sports-related task specific dystonia | Treatment | Behavioral Therapy | NA | Overall Symptoms (subjective) | All patients improved | IV | |
| N | 4 | Details | Standardized behavioral therapy & relaxation techniques | |||||||
| M/F | 3/1 | |||||||||
| Age (y) | 40, 53, 49, 19 | Frequency/Duration | 8 sessions, 16 w | |||||||
| Kobori et al. [108] Japan | Case report | Type | Sports-related task specific dystonia | Treatment | Behavioral therapy | NA | Numerical rating scale (pain) | Improvement in pain | IV | |
| N | 1 | Details | Psychoeducation, behavioral therapy, cognitive restructuring | |||||||
| M/F | M | |||||||||
| Age (y) | 21 | Frequency/Duration | 7 sessions, 8 m | |||||||
| Wieck et al. [109] UK | RCT | Type | Writer’s cramp | Treatment | Habit reversal treatment | Relaxation training | Writing tasks (performance) | Both groups significantly improved writing performance No significant difference between groups | II | |
| N | 9 | 11 | Details | Putting pen down & contract opposing muscle when cramp | ||||||
| M/F | 8/1 | 8/3 | ||||||||
| Age (y) | 52 ± 10 | 49 ± 16 | Frequency/Duration | 5 sessions, 4 w | ||||||
| Functional dystonia | ||||||||||
| Hsieh et al. [110] UK | Case report | N | 1 | Treatment | Multidisciplinary program | NA | Walking test, BDI, Work and Social Adjustment Scale (performance, depression, ADL) | Significant improvement in all outcomes | IV | |
| M/F | F | Details | Cognitive, behavioral therapy, PT, physical activities, psychoeducation | |||||||
| Age (y) | Early 40s | Frequency/Duration | 3 d/w, 8w | |||||||
| Khachane et al. [111] Australia | Case report | N | 1 | Treatment | Mind-Body program | NA | Overall Symptoms (subjective) | Full recovery | IV | |
| M/F | M | Details | Sleep hygiene, hypnosis, psychotherapy, PT, OT | |||||||
| Age (y) | 14 | Frequency/Duration | 3 w admission | |||||||
| Chudleigh et al. [112] Australia | Case report | N | 1 | Treatment | Multidisciplinary care | NA | Overall Symptoms (subjective) | Full recovery | IV | |
| M/F | F | Details | Child psychiatrist, psychologist, nurse consultant & pediatric resident | |||||||
| Age (y) | 17 | Frequency/Duration | 2 session/w, 3 w | |||||||
| Chatterjee et al. [113] India | Case report | N | 1 | Treatment | Structured psychotherapy and family intervention | NA | Overall Symptoms (subjective) | Full recovery | IV | |
| M/F | M | Details | NA | |||||||
| Age (y) | 13 | Frequency/Duration | NA | |||||||
| Puccioni-Sohler et al. [114] Brazil | Case report | N | 1 | Treatment | Psychotherapy | NA | Overall Symptoms (subjective) | Improvement of the involuntary movement | IV | |
| M/F | M | Details | NA | |||||||
| Age (y) | 46 | Frequency/Duration | NA | |||||||
Behavioral therapy for dystonia.
Values are shown as mean (standard deviation or SD), if SD was not available in the article min - max were shown if possible. AAN class, American Academy of Neurology classification framework; number of patients, n; male, M; female, F; year, y; month, week, w; hour, h; minute, min; second, s; not applicable, NA; Randomized controlled trial, RCT; electromyography, EMG; Range of Motion, ROM; cognitive behavioral therapy, CBT; Physical Therapy, PT; Occupational therapy, OT; Beck's Depression Inventory, BDI; Activities of Daily Living, ADL.
In WC, Wieck A. et al. conducted a randomized study assigning 23 patients to either 4 weeks of habit reversal training or relaxation therapy. They found that both treatment groups experienced a similar degree of improvement in writing performance, suggesting that the benefits observed may not be specific to habit reversal training alone [109]. Tibben et al. investigated the effectiveness of standardized behavioral therapy and relaxation techniques, including hypnosis, in four patients with sports-related TSD. After 16 weeks of therapy, all participants reported subjective improvements, suggesting a potential benefit of these interventions, though larger studies are needed to confirm these findings [107]. Another report found behavioral therapy as possibly effective in improving pain in sports-related TSD [108].
In functional dystonia, published reports are limited. Khachane et al. described a case in which a three-week multidisciplinary mind-body program, including sleep hygiene, hypnosis, psychotherapy, PT, and OT, led to a full recovery in a 14-year-old boy with rapidly worsening generalized dystonia [111]. Chudleigh et al. reported that multidisciplinary care involving a child psychiatrist, psychologist, nurse consultant, and pediatric resident led to the complete resolution of symptoms in a patient with functional dystonia [112]. Hsieh et al. reported a case in which a multidisciplinary program consisting of PT, CBT, and psychoeducation led to improvements in motor performance and activities of daily living [110]. Chatterjee et al. and Puccioni-Sohler et al. reported that psychotherapy led to subjective symptom improvement in individual cases of functional dystonia (presenting as facial dystonia, CD, focal limb dystonia, and generalized dystonia) [113, 114].
Recommendation
Habit reversal training as well as relaxation therapy can improve symptoms of WC. Behavioral therapy and relaxation techniques, including hypnosis, may help alleviate subjective symptoms and pain in sports-related TSD. In functional dystonia, a multidisciplinary approach incorporating psychotherapy, PT, and OT to address mind-body training, such as hypnosis, CBT and sleep hygiene, shows promise, however further studies are needed to establish sustained efficacy.
Discussion
Principles underlying rehab therapy
It is essential to first understand the pathophysiology of dystonia before exploring the principles underlying rehabilitation therapy. In dystonia, there is a loss of inhibition of neural signal processing occurring at multiple levels, from the motor cortex to the brainstem to the spinal cord. A loss of inhibitory control results in excessive contractions, co-contractions, and the overflow of muscle activity. Another core pathophysiological mechanism for dystonia is abnormalities in sensory processing, or in “sensorimotor integration.” Dystonia is not only a motor disorder but also a sensory disorder [115]. The third important mechanism is related to brain plasticity. The human brain, as we understand today, is indeed plastic and capable of learning new motor behaviors through synaptic changes within neural circuits. In dystonia, however, the regulation of sensorimotor plasticity is found to be aberrant, excessive, and maladaptive [115]. When plasticity changes in neural synapses are excessive, disorganized, and exceed the boundaries of normal homeostatic mechanisms, abnormal movement patterns develop. These abnormal motor engrams and subroutines become stored in the motor cortex and are activated by genetic predispositions and/or environmental triggers [2].
Rehabilitation interventions could plausibly target and correct multiple pathophysiological mechanisms underlying dystonia. A core principle of rehabilitation is intensive motor training, to restore balance between agonists and antagonists. In CD, this strategy includes relaxing overactive dystonic muscles while strengthening compensatory muscles to produce opposing movements, with or without biofeedback to modulate muscle activation. In TSDs, task specific motor training can potentially improve functional performance. Fixed deformity in dystonia can develop due to prolonged abnormal posturing, muscle contractures, secondary changes in soft tissues, and structural remodeling of joints over time. Early introduction of rehab could potentially prevent or improve the development of these fixed deformities associated with dystonia. Then intensive sensory training could enhance sensory discrimination abilities or the use of controlled sensory deprivation in the affected body part, both of which may promote somatosensory reorganization and contribute to motor improvement. Compensatory strategies with or without modalities or assistive devices, to help individuals adapt to their impairments by utilizing unaffected body parts or modifying movement patterns. Last but not the least is the potential application of motor training protocols to promote normal adaptive plasticity, replacing abnormal motor engrams and subroutines with more appropriate motor programs.
Rehab in focal cervical dystonia
In CD, while only some studies provide moderate or high-quality evidence, combining PT exercises with BoNT appears promising. As some muscles excessively contract, causing involuntary jerky movements of the neck, PT strategies focus on relaxing these overactive muscles while strengthening underactive muscles, incorporating posture education, stretching, massage, and biofeedback-based motor activation to restore balanced muscle function and improve neck alignment. Furthermore, the use of kinesiotape or vibrotactile stimulation can aid in less pain by providing sensory feedback, reducing muscle overactivity, and enhancing proprioception. Whether PT is administered with a standardized approach (which can be implemented broadly across the clinic population) or is individualized (which improves patient engagement and compliance), the clinical improvements reported so far appear to be similar. However, further data is needed to determine the most effective, timing, frequency, duration and intensity of therapy to fully understand the long-term benefits.
Rehab in focal limb dystonia
The rehabilitation literature on focal limb dystonia has primarily focused on TSDs such as WC, MD, and sports-related dystonia. Many studies have employed immobilization-based strategies, either by restricting unaffected digits or body parts using splints or taping to encourage adaptation and retraining of movement patterns (sensorimotor retuning) or by immobilizing the affected body part (sensory deprivation). The goal is to promote somatosensory reorganization and improve motor function. Other studies focused on specific sensory discrimination protocols or learning based sensorimotor training; however, the results so far have been mixed.
Rehab in functional dystonia
Functional neurological disorders are clinical syndromes characterized by neurological symptoms and deficits that suggest dysfunction of the nervous system. A hallmark feature of these disorders is variability in performance, both when attempting the same task repeatedly and across different tasks. Although consensus statements have been developed to guide the treatment of functional motor disorders [116, 117], there are currently no universally established treatment protocols specifically addressing rehabilitation or exercise therapy for functional dystonia. While PT, OT, speech therapy, and psychotherapy are frequently prescribed in clinical practice, evidence of their efficacy remains limited. Multidisciplinary care, particularly incorporating CBT and psychotherapy, or mind-body programs, appears to be a promising intervention for managing dystonia. Key rehabilitation strategies should include patient education about their symptoms, movement retraining to shift attention away from abnormal movements, and a gradual approach to fostering the patient’s belief that they are capable of normal movement, which may enhance functional outcomes and motor control [118]. Recommended rehabilitations for individual dystonia according to distribution are shown in Figure 2.
FIGURE 2
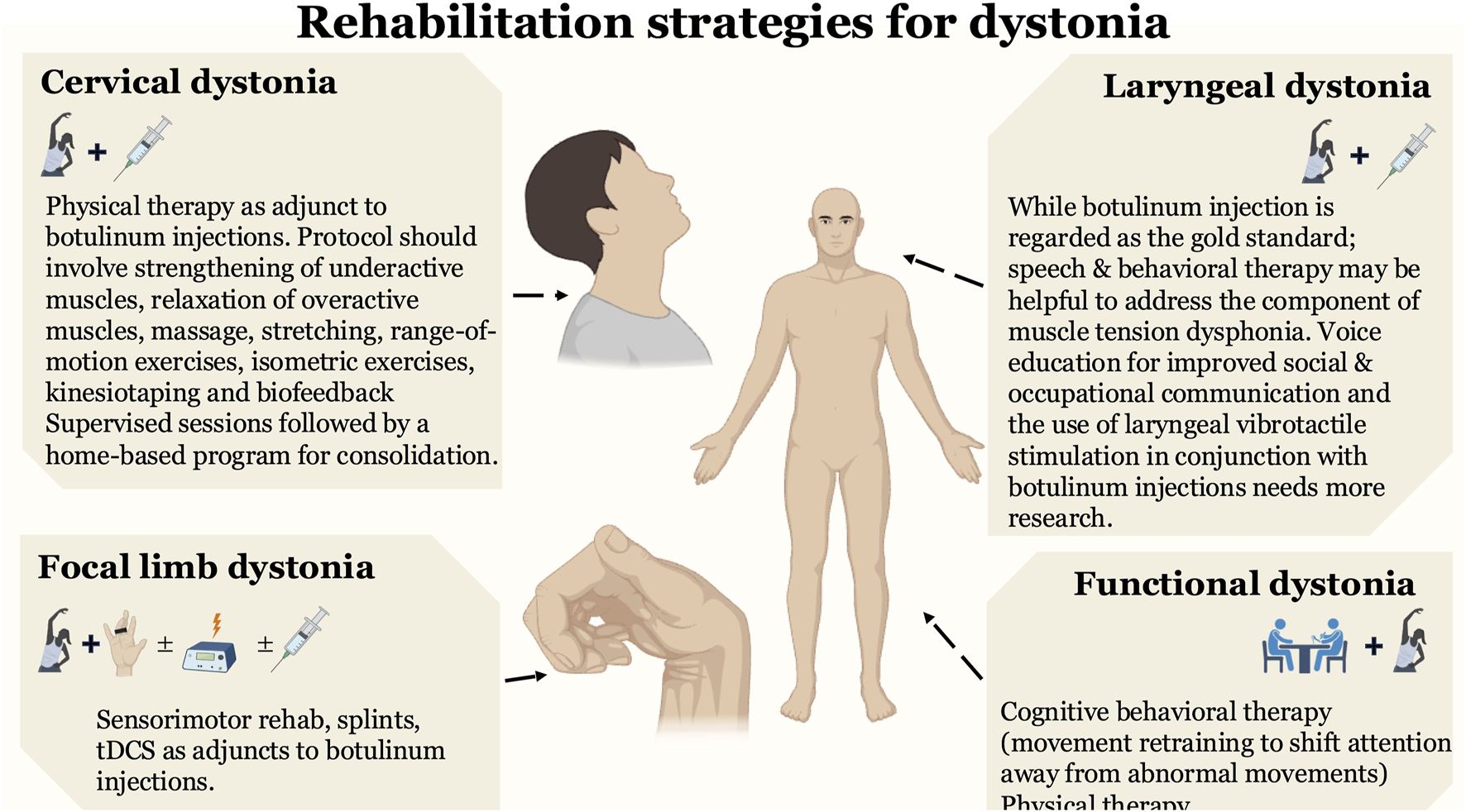
Rehabilitation strategies promising for dystonia. The figure highlights rehabilitation strategies that are most effective and practical for various forms of dystonia, including focal cervical dystonia, focal limb dystonia (task-specific dystonia), and laryngeal dystonia. We recommend utilizing these strategies alongside botulinum toxin injections as adjunct therapies. For functional dystonia, although evidence is limited, we suggest a combination of cognitive behavioral therapy and physical therapy.
Scope in other dystonias
There is limited evidence on the role of rehabilitation in many focal dystonias, such as blepharospasm, oromandibular dystonia, LD, and lower limb dystonia. Aside from a few case reports involving functional dystonia with generalized features, it remains unclear whether rehabilitation therapies can be broadly applied. Generalized dystonia, particularly genetic forms, tends to be the most severe, often beginning in childhood and commonly necessitating surgical interventions. At some movement disorder centers, rehab-based therapies are given due consideration before and after DBS in patients with segmental and generalized dystonia with the goal of improving specific regional symptoms and functional outcomes. However, studies demonstrating sustained and quantifiable benefits are currently lacking, and it remains unclear whether these interventions could serve as effective adjuncts to accelerate the clinical response to DBS (dystonia improvement can be slow and needing several months in many patients) or aid in the long-term maintenance of functional gains achieved through DBS (concerns for secondary worsening) [119].
Challenges for rehab research
Several challenges, well-summarized in a previous systematic review [15], warrant reiteration. These include the extensive commitment required for rehabilitation interventions, with the lack of supervision in exercise programs potentially leading to compliance issues. Due to the extremely rare nature of some forms of dystonia, many studies have small sample sizes, making interpretation and generalization difficult. Collective efforts, such as multicenter investigations, help increase patient numbers, but it is essential to ensure consistency in treatment methods across different centers to maintain the reliability and validity of the study findings. Additionally, implementing appropriate control conditions is challenging, as there is no true placebo equivalent; some studies use alternative interventions like stretching, massage, educational sessions, or home exercises as controls, but these may still provide benefits, complicating the isolation of the specific efficacy of the experimental treatment. Finally validated and standardized scales that can accurately capture effect sizes, minimal clinically important difference and minimal detectable changes should be implemented. Patient-reported outcomes are valuable for capturing the patient’s perspective on symptom severity, functional limitations, and QoL. Subjective assessments such as the Visual Analogue Scale, Global Rating of Change, and Goal Attainment Scale can be used to evaluate treatment effectiveness and individual progress. These tools help measure patient-perceived improvements, ensuring a comprehensive understanding of rehabilitation outcomes beyond objective clinical assessments.
To conclude, rehabilitation strategies encompassing progressive motor, or sensorimotor training, have the capability to enhance (could even augment) and sustain benefits of pharmacological or neuromodulation therapies. Neuroplasticity-driven techniques, including sensorimotor retraining, retuning and biofeedback, can help maximize and prolong functional improvements by reinforcing adaptive motor patterns. Multimodal interventions (e.g., combining PT with CBT) are particularly helpful for managing challenging conditions like functional dystonia. Regular follow-ups and individually tailored adjustments in therapy plans can further support long-term benefits. Home-based reinforcement exercises can maintain gains made in supervised settings.
Statements
Author contributions
HK: Conception, Organization, Execution, Writing of the first draft. KN: Review, and Critique. LW: Review, and Critique. AK: Review, and Critique. AW: Conception, Organization, Execution, Review, and Critique. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by KAKENHI 23K19409, 22K10863, NIH R01NS122943, and NS121120-01. HK received grant support from the Japan Society for the Promotion of Science KAKENHI, and the Japanese Society of Neurology. KN received grant support from the Japan Society for the Promotion of Science KAKENH. AW reports grant support from the NIH R01NS122943 as PI and Ro1 NS121120-01 as a Co-I. She reports past funding from Benign Essential Blepharospasm Research foundation, Dystonia coalition, Dystonia Medical Research foundation, National Organization for Rare Disorders. AS has received consultant fees from Merz, Jazz and Acadia. She is the current Vice President for the Tremor Research Group and recent advisor for Supernus and Biogen-Sage.
Conflict of interest
Author KN was employed by Sunwels Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
AlbaneseABhatiaKBressmanSBDelongMRFahnSFungVSCet alPhenomenology and classification of dystonia: a consensus update. Mov Disord (2013) 28(7):863–73. 10.1002/mds.25475
2.
Wagle ShuklaA. Basis of movement control in dystonia and why botulinum toxin should influence it?Toxicon (2024) 237:107251. 10.1016/j.toxicon.2023.107251
3.
JunkerJHallJBermanBDVidailhetMRozeEBäumerTet alLongitudinal predictors of health-related quality of life in isolated dystonia. J Neurol (2024) 271(2):852–63. 10.1007/s00415-023-12022-4
4.
JunkerJBermanBDHallJWahbaDWBrandtVPerlmutterJSet alQuality of life in isolated dystonia: non-motor manifestations matter. J Neurol Neurosurg Psychiatry (2021) 92:622–8. 10.1136/jnnp-2020-325193
5.
FreyJRamirez-ZamoraAWagle ShuklaA. Applications of transcranial magnetic stimulation for understanding and treating dystonia. Adv Neurobiol (2023) 31:119–39. 10.1007/978-3-031-26220-3_7
6.
AyoubN. Botulinum toxin therapy: a comprehensive review on clinical and pharmacological insights. J Clin Med (2025) 14(6):2021. 10.3390/jcm14062021
7.
HallettMAlbaneseADresslerDSegalKRSimpsonDMTruongDet alEvidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon (2013) 67:94–114. 10.1016/j.toxicon.2012.12.004
8.
TsuboiTJabarkheelZFooteKDOkunMSWagle ShuklaA. Importance of the initial response to GPi deep brain stimulation in dystonia: a nine year quality of life study. Parkinsonism Relat Disord (2019) 64:249–55. 10.1016/j.parkreldis.2019.04.024
9.
DelnoozCCHorstinkMWTijssenMAvan de WarrenburgBP. Paramedical treatment in primary dystonia: a systematic review. Mov Disord (2009) 24(15):2187–98. 10.1002/mds.22608
10.
HikaruKKoichiNLAlisonWMichaelRKAparnaSOShuklaW. Rehabilitation strategies for sleep and autonomic symptoms in Parkinson's disease. Curr Neurol Neurosci Rep (2025) 24. 10.1007/s11910-025-01433-7
11.
Cologne. InformedHealth.org. In brief: physical therapy. [Updated 2024 Mar 19].
12.
Cologne IoI. In brief: What is occupational therapy? Institute for quality and efficiency in health care (IQWiG) (2006). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK561515/ (Accessed October, 2024).
13.
HautekietARaesKGeersSSantensPOostraK. Evidence of rehabilitation therapy in task-specific focal dystonia: a systematic review. Eur J Phys Rehabil Med Mese (2021) 57(0):710–9. 10.23736/s1973-9087.21.06677-6
14.
StallardP. Evidence-based practice in cognitive-behavioural therapy. Arch Dis Child (2022) 107(2):109–13. 10.1136/archdischild-2020-321249
15.
PrudenteCNZetterbergLBringABradnamLKimberleyTJ. Systematic review of rehabilitation in focal dystonias: classification and recommendations. Mov Disord Clin Pract (2018) 5(3):237–45. 10.1002/mdc3.12574
16.
BletonJPCosséCCaloc'hTSuarez MorenoADiverresEDerkinderenPet alCombination of anodal tDCS of the cerebellum with a goal-oriented motor training to treat cervical dystonia: a pilot case series. Front Neurol (2024) 15:1381390. 10.3389/fneur.2024.1381390
17.
Dec-ĆwiekMPorębskaKSawczyńskaKKubalaMWitkowskaMZmijewskaKet alKinesioTaping after botulinum toxin type A for cervical dystonia in adult patients. Brain Behav (2022) 12(4):e2541. 10.1002/brb3.2541
18.
CastagnaACaronniACrippaASciumèLGiacobbiGCorriniCet alSensorimotor perceptive rehabilitation integrated (SPRInt) program: exercises with augmented movement feedback associated to botulinum neurotoxin in idiopathic cervical dystonia-an observational study. Neurol Sci (2020) 41(1):131–8. 10.1007/s10072-019-04061-5
19.
WernerCDerlienSBestNWitteOSmolenskiUCGüntherA. Effects of a 3-month physiotherapy intervention in stable cervical dystonia as add-on to botulinum toxin therapy. Physikalische Medizin, Rehabilitationsmedizin, Kurortmedizin (2019) 29(01):53–7. 10.1055/a-0756-9954
20.
HuWRundle-GonzalezVKulkarniSJMartinez-RamirezDAlmeidaLOkunMSet alA randomized study of botulinum toxin versus botulinum toxin plus physical therapy for treatment of cervical dystonia. Parkinsonism Relat Disord (2019) 63:195–8. 10.1016/j.parkreldis.2019.02.035
21.
QueirozMAChienHFSekeff-SallemFABarbosaER. Physical therapy program for cervical dystonia: a study of 20 cases. Funct Neurol (2012) 27(3):187–92.
22.
CounsellCSinclairHFowlieJTyrrellEDerryNMeagerPet alA randomized trial of specialized versus standard neck physiotherapy in cervical dystonia. Parkinsonism Relat Disord (2016) 23:72–9. 10.1016/j.parkreldis.2015.12.010
23.
TassorelliCManciniFBalloniLPacchettiCSandriniGNappiGet alBotulinum toxin and neuromotor rehabilitation: an integrated approach to idiopathic cervical dystonia. Mov Disord (2006) 21(12):2240–3. 10.1002/mds.21145
24.
RamdharryG. Case report: physiotherapy cuts the dose of botulinum toxin. Physiother Res Int (2006) 11(2):117–22. 10.1002/pri.326
25.
StankovićIČolovićHŽivkovićVStamenovicJStankovicAZlatanovicDet alThe effect of physical therapy in the treatment of patients with cervical dystonia with or without concomitant use of botulinum toxin. Vojnosanitetski pregled. (2018) 75(10):1035–40. 10.2298/vsp161115016s
26.
El-BahrawyMNEl-TamawyMSShalabyNMAbdel-AlimAM. Cervical dystonia: abnormal head posture and its relation to hand function. Egypt J Neurol Psychiatr Neurosurg (2009) 46:203–8.
27.
van den DoolJVisserBKoelmanJHEngelbertRHTijssenMA. Long-term specialized physical therapy in cervical dystonia: outcomes of a randomized controlled trial. Arch Phys Med Rehabil (2019) 100(8):1417–25. 10.1016/j.apmr.2019.01.013
28.
de Oliveira SouzaCGoulardinsJCoelhoDBCasagrandeSContiJLimongiJCPet alNon-invasive brain stimulation and kinesiotherapy for treatment of focal dystonia: instrumental analysis of three cases. J Clin Neurosci (2020) 76:208–10. 10.1016/j.jocn.2020.04.025
29.
BradnamLVMcDonnellMNRiddingMC. Cerebellar intermittent theta-burst stimulation and motor control training in individuals with cervical dystonia. Brain Sci (2016) 6(4):56. 10.3390/brainsci6040056
30.
GildenbergPL. Comprehensive management of spasmodic torticollis. Appl Neurophysiol (1981) 44(4):233–43. 10.1159/000102206
31.
CairnsSLLeBowMD. Meige's disease misdiagnosed as anxiety disorder. J Behav Ther Exp Psychiatry (1991) 22(3):221–3. 10.1016/0005-7916(91)90020-6
32.
SilvermanEPGarvanCShrivastavRSapienzaCM. Combined modality treatment of adductor Spasmodic dysphonia. J Voice (2012) 26(1):77–86. 10.1016/j.jvoice.2010.08.004
33.
MurryTWoodsonGE. Combined-modality treatment of adductor Spasmodic dysphonia with botulinum toxin and voice therapy. J Voice (1995) 9(4):460–5. 10.1016/s0892-1997(05)80211-5
34.
Rosset-LlobetJFàbregas-MolasSPascual-LeoneÁ. Effect of transcranial direct current stimulation on neurorehabilitation of task-specific dystonia: a double-blind, randomized clinical trial. Med Probl Perform Art (2015) 30(3):178–84. 10.21091/mppa.2015.3033
35.
FuruyaSNitscheMAPaulusWAltenmüllerE. Surmounting retraining limits in musicians' dystonia by transcranial stimulation. Ann Neurol (2014) 75(5):700–7. 10.1002/ana.24151
36.
ButtkusFBaurVJabuschHCde la Cruz Gomez-PellinMPaulusWNitscheMAet alSingle-session tDCS-supported retraining does not improve fine motor control in musician's dystonia. Restor Neurol Neurosci (2011) 29(2):85–90. 10.3233/rnn-2011-0582
37.
ButtkusFBaurVJabuschHCPaulusWNitscheMAAltenmüllerE. Retraining and transcranial direct current stimulation in musician's dystonia - a case report. Mov Disord (2010) 25(11):1758–60. 10.1002/mds.23259
38.
VizcarraJALopez-CastellanosJRDwivediAKSchmerlerDARiesSEspayAJ. OnabotulinumtoxinA and cognitive behavioral therapy in functional dystonia: a pilot randomized clinical trial. Parkinsonism Relat Disord (2019) 63:174–8. 10.1016/j.parkreldis.2019.02.009
39.
GiorgiVApostoloGBertelèL. Treating dystonia in a soccer player through an integrated rehabilitative approach: a case report. J Sport Rehabil (2024) 33(5):365–75. 10.1123/jsr.2023-0100
40.
MajumdarALópez-CasasJPooPColomerJGalvanMLingappaLet alSyndrome of fixed dystonia in adolescents--short term outcome in 4 cases. Eur J Paediatr Neurol (2009) 13(5):466–72. 10.1016/j.ejpn.2008.09.005
41.
LeeAJahnkeAKAltenmüllerE. Fixed dystonia of the left hand in a violinist: a rare functional disorder. Tremor Other Hyperkinet Mov (N Y) (2013) 3:03. 10.5334/tohm.151
42.
AntelmiEContiECarecchioMTinazziM. Functional and idiopathic cervical dystonia in two family members: a challenging diagnosis. Tremor Other Hyperkinet Mov (N Y) (2020) 10:51. 10.5334/tohm.558
43.
KimberleyTJBorichMRSchmidtRLCareyJRGillickB. Focal hand dystonia: individualized intervention with repeated application of repetitive transcranial magnetic stimulation. Arch Phys Med Rehabil (2015) 96(4 Suppl. l):S122–8. 10.1016/j.apmr.2014.07.426
44.
ZieglerJSvon StauffenbergMVlahoSBöhlesHKieslichM. Dystonia with secondary contractures: a psychogenic movement disorder mimicking its neurological counterpart. J Child Neurol (2008) 23(11):1316–8. 10.1177/0883073808318060
45.
BeylergilSBMukundaKNElkasabyMPerlmutterJSFactorSBäumerTet alTremor in cervical dystonia. Dystonia (2024) 3:11309. 10.3389/dyst.2024.11309
46.
PandeySSharmaS. Meige's syndrome: history, epidemiology, clinical features, pathogenesis and treatment. J Neurol Sci (2017) 372:162–70. 10.1016/j.jns.2016.11.053
47.
SimonyanKBarkmeier-KraemerJBlitzerAHallettMHoudeJFJacobson KimberleyTet alLaryngeal dystonia: multidisciplinary update on terminology, pathophysiology, and research priorities. Neurology (2021) 96(21):989–1001. 10.1212/wnl.0000000000011922
48.
RicciardiLBolognaMMarsiliLEspayAJ. Dysfunctional networks in functional dystonia. Adv Neurobiol (2023) 31:157–76. 10.1007/978-3-031-26220-3_9
49.
KaseK. Clinical therapeutic applications of the kinesio (! R) taping method. Albuquerque (2003).
50.
KimberleyTJSchmidtRLChenMDykstraDDBuetefischCM. Mixed effectiveness of rTMS and retraining in the treatment of focal hand dystonia. Front Hum Neurosci (2015) 9:385. 10.3389/fnhum.2015.00385
51.
Isabel Useros-OlmoAPhDPTMartínez-PerníaDHuepeDP. The effects of a relaxation program featuring aquatic therapy and autogenic training among people with cervical dystonia (a pilot study). Physiother Theor Pract (2020) 36(4):488–97. 10.1080/09593985.2018.1488319
52.
BoyceMJCanningCGMahantNMorrisJLatimerJFungVS. Active exercise for individuals with cervical dystonia: a pilot randomized controlled trial. Clin Rehabil (2013) 27(3):226–35. 10.1177/0269215512456221
53.
ZetterbergLHalvorsenKFärnstrandCAquiloniusSMLindmarkB. Physiotherapy in cervical dystonia: six experimental single-case studies. Physiother Theor Pract (2008) 24(4):275–90. 10.1080/09593980701884816
54.
SmaniaNCoratoETinazziMMontagnanaBFiaschiAAgliotiSM. The effect of two different rehabilitation treatments in cervical dystonia: preliminary results in four patients. Funct Neurol (2003) 18(4):219–25.
55.
DuddyJMcLellanD. Lack of influence of EMG biofeedback in relaxation training for spasmodic torticollis. Clin Rehabil (1995) 9(4):297–303. 10.1177/026921559500900404
56.
SpencerJGoetschVLBrugnoliRJHermanS. Behavior therapy for spasmodic torticollis: a case study suggesting a causal role for anxiety. J Behav Ther Exp Psychiatry (1991) 22(4):305–11. 10.1016/0005-7916(91)90049-b
57.
LeplowB. Heterogeneity of biofeedback training effects in spasmodic torticollis: a single-case approach. Behav Res Ther (1990/01/01/1990) 28(4):359–65. 10.1016/0005-7967(90)90091-V
58.
JahanshahiMSartoryGMarsdenCD. EMG biofeedback treatment of torticollis: a controlled outcome study. Biofeedback Self Regul (1991) 16(4):413–48. 10.1007/bf00999994
59.
CleelandCS. Behavioral technics in the modification of spasmodic torticollis. Neurology (1973) 23(11):1241–7. 10.1212/wnl.23.11.1241
60.
MartinPR. Spasmodic torticollis: investigation and treatment using EMG feedback training. Behav Ther (1981/03/01/1981) 12(2):247–62. 10.1016/S0005-7894(81)80076-7
61.
BrudnyJKoreinJGrynbaumBBFriedmannLWWeinsteinSSachs-FrankelGet alEMG feedback therapy: review of treatment of 114 patients. Arch Phys Med Rehabil (1976) 57(2):55–61. 10.1007/BF00999994
62.
KeatleyAWirzS. Is 20 years too long? Improving intelligibility in long-standing dysarthria--a single case treatment study. Eur J Disord Commun (1994) 29(2):183–201. 10.3109/13682829409041491
63.
AckermannBAltenmüllerE. The development and use of an anatomy-based retraining program (MusAARP) to assess and treat focal hand dystonia in musicians-A pilot study. J Hand Ther (2021) 34(2):309–14. 10.1016/j.jht.2021.05.007
64.
ButlerKSadnickaAFreemanJMeppelinkAMPareésIMarsdenJet alSensory–motor rehabilitation therapy for task-specific focal hand dystonia: a feasibility study. Hand Ther (2018) 23(2):53–63. 10.1177/1758998318764219
65.
YoshieMSakaiNOhtsukiTKudoK. Slow-down exercise reverses sensorimotor reorganization in focal hand dystonia: a case study of a pianist. Int J Neurorehabil (2015) 2(2):2376–0281. 10.1016/s0894-1130(00)80021-6
66.
HashimotoYOtaTMukainoMLiuMUshibaJ. Functional recovery from chronic writer's cramp by brain-computer interface rehabilitation: a case report. BMC Neurosci (2014) 15:103. 10.1186/1471-2202-15-103
67.
ChengFPGroßbachMAltenmüllerEO. Altered sensory feedbacks in pianist's dystonia: the altered auditory feedback paradigm and the glove effect. Front Hum Neurosci (2013) 7:868. 10.3389/fnhum.2013.00868
68.
de LisleRSpeedyDBThompsonJ. Rehabilitation of a cellist whose vibrato was affected by focal dystonia. Med Probl Performing Artists (2012) 27(4):227–30. 10.21091/mppa.2012.4042
69.
BaurBFürholzerWMarquardtCHermsdörferJ. Auditory grip force feedback in the treatment of Writer's cramp. J Hand Ther (2009) 22(2):163–71. 10.1016/j.jht.2008.11.001
70.
BylNNArcherESMcKenzieA. Focal hand dystonia: effectiveness of a home program of fitness and learning-based sensorimotor and memory training. J Hand Ther (2009) 22(2):183–98. 10.1016/j.jht.2008.12.003
71.
McKenzieALGoldmanSBarrangoCShrimeMWongTBylN. Differences in physical characteristics and response to rehabilitation for patients with hand dystonia: musicians' cramp compared to writers' cramp. J Hand Ther (2009) 22(2):172–82. 10.1016/j.jht.2008.12.006
72.
BergerHJvan der WerfSPHorstinkCACoolsAROyenWJHorstinkMW. Writer's cramp: restoration of striatal D2-binding after successful biofeedback-based sensorimotor training. Parkinsonism Relat Disord (2007) 13(3):170–3. 10.1016/j.parkreldis.2006.09.003
73.
de LisleRSpeedyDBThompsonJMDMauriceDG. Effects of pianism retraining on three pianists with focal dystonia. Med Probl Performing Artists (2006) 21(3):105–11. 10.21091/mppa.2006.3022
74.
SakaiN. Slow-down exercise for the treatment of focal hand dystonia in pianists. Med Probl Performing Artists (2006) 21(1):25–8. 10.21091/mppa.2006.1005
75.
SchenkTBauerBSteidleBMarquardtC. Does training improve writer's cramp? An evaluation of a behavioral treatment approach using kinematic analysis. J Hand Ther (2004) 17(3):349–63. 10.1197/j.jht.2004.04.005
76.
BylNNNagajaranSMcKenzieAL. Effect of sensory discrimination training on structure and function in patients with focal hand dystonia: a case series. Arch Phys Med Rehabil (2003) 84(10):1505–14. 10.1016/s0003-9993(03)00276-4
77.
ZeunerKEBara-JimenezWNoguchiPSGoldsteinSRDambrosiaJMHallettM. Sensory training for patients with focal hand dystonia. Ann Neurol (2002) 51(5):593–8. 10.1002/ana.10174
78.
BylNNMcKenzieA. Treatment effectiveness for patients with a history of repetitive hand use and focal hand dystonia: a planned, prospective follow-up study. J Hand Ther (2000) 13(4):289–301. 10.1016/s0894-1130(00)80021-6
79.
DeepakKKBehariM. Specific muscle EMG biofeedback for hand dystonia. Appl Psychophysiol Biofeedback (1999) 24(4):267–80. 10.1023/a:1022239014808
80.
O'NeillMAGwinnKAAdlerCH. Biofeedback for writer's cramp. Am J Occup Ther (1997) 51(7):605–7. 10.5014/ajot.51.7.605
81.
GrosPBhattHGilmourGSLidstoneSC. Rehabilitation for functional dystonia: cases and review of the literature. Mov Disord Clin Pract (2024) 11(8):1018–24. 10.1002/mdc3.14121
82.
StephenCDSharmaNCallahanJCarsonAJPerezDL. A case of functional dystonia with associated functional neurological symptoms: diagnostic and therapeutic challenges. Harv Rev Psychiatry (2017) 25(5):241–51. 10.1097/hrp.0000000000000135
83.
XuJCostanzoMAvanzinoLMartinoDSalehiPStandalSet alVibro-tactile stimulation of the neck reduces pain in people with cervical dystonia: a proof-of-concept study. Neurol Sci (2024) 45(10):4847–56. 10.1007/s10072-024-07561-1
84.
PelosinEAvanzinoLMarcheseRStramesiPBilanciMTrompettoCet alKinesiotaping reduces pain and modulates sensory function in patients with focal dystonia: a randomized crossover pilot study. Neurorehabil Neural Repair (2013) 27(8):722–31. 10.1177/1545968313491010
85.
KhosravaniSMahnanAYehILAmanJEWatsonPJZhangYet alLaryngeal vibration as a non-invasive neuromodulation therapy for Spasmodic dysphonia. Sci Rep (2019) 9(1):17955. 10.1038/s41598-019-54396-4
86.
BraviRIoannouCIMinciacchiDAltenmüllerE. Assessment of the effects of kinesiotaping on musical motor performance in musicians suffering from focal hand dystonia: a pilot study. Clin Rehabil (2019) 33(10):1636–48. 10.1177/0269215519852408
87.
OborzyńskiJGajosAMajosAKujawaJStefańczykLBoguckiA. Motor re-training and immobilisation in the treatment of writer’s cramp: a clinical and fMRI study. Aktualności Neurologiczne. 10/15 (2018) 18:68–73. 10.15557/AN.2018.0009
88.
BarrettMJBressmanSBLevyOAFahnSO'DellMW. Functional electrical stimulation for the treatment of lower extremity dystonia. Parkinsonism Relat Disord (2012) 5:660–1. 10.1016/j.parkreldis.2011.09.017
89.
SingamNVDwivediAEspayAJ. Writing orthotic device for the management of writer's cramp. Front Neurol (2013) 4:2. 10.3389/fneur.2013.00002
90.
WaissmanFQOrsiniMNascimentoOJLeiteMAPereiraJS. Sensitive training through body awareness to improve the writing of patients with writer's cramp. Neurol Int (2013) 5(4):e24. 10.4081/ni.2013.e24
91.
BerquePGrayHMcFadyenA. A combination of constraint-induced therapy and motor control retraining in the treatment of focal hand dystonia in musicians: a long-term follow-up study. Med Probl Perform Art (2013) 28(1):33–46. 10.21091/mppa.2013.1007
92.
Rosset-LlobetJFàbregas-MolasS. Long-term treatment effects of sensory motor retuning in a pianist with focal dystonia. Med Probl Perform Art (2011) 26(2):106–7. 10.21091/mppa.2011.2016
93.
MeunierSBletonJPMazevetDSanglaSGrabliDRozeEet alTENS is harmful in primary writing tremor. Clin Neurophysiol (2011) 122(1):171–5. 10.1016/j.clinph.2010.06.012
94.
WaissmanFQPereiraJSNascimentoOJ. A new therapeutic proposal for writer's cramp: a case report. Sao Paulo Med J (2010) 128(2):96–8. 10.1590/s1516-31802010000200010
95.
BerquePGrayHHarknessCMcFadyenA. A combination of constraint-induced therapy and motor control retraining in the treatment of focal hand dystonia in musicians. Med Probl Perform Art (2010) 25(4):149–61. 10.21091/mppa.2010.4032
96.
TrompettoCAvanzinoLBoveMMarinelliLMolfettaLTrentiniRet alExternal shock waves therapy in dystonia: preliminary results. Eur J Neurol (2009) 16(4):517–21. 10.1111/j.1468-1331.2008.02525.x
97.
ZeunerKEPellerMKnutzenAHallettMDeuschlGSiebnerHR. Motor re-training does not need to be task specific to improve writer's cramp. Mov Disord (2008) 23(16):2319–27. 10.1002/mds.22222
98.
TinazziMZarattiniSValerianiMStanzaniCMorettoGSmaniaNet alEffects of transcutaneous electrical nerve stimulation on motor cortex excitability in writer's cramp: neurophysiological and clinical correlations. Mov Disord (2006) 21(11):1908–13. 10.1002/mds.21081
99.
TinazziMFarinaSBhatiaKFiaschiAMorettoGBertolasiLet alTENS for the treatment of writer's cramp dystonia: a randomized, placebo-controlled study. Neurology (2005) 64(11):1946–8. 10.1212/01.wnl.0000163851.70927.7e
100.
ZeunerKEShillHASohnYHMolloyFMThorntonBCDambrosiaJMet alMotor training as treatment in focal hand dystonia. Mov Disord (2005) 20(3):335–41. 10.1002/mds.20314
101.
PesentiABarbieriSPrioriA. Limb immobilization for occupational dystonia: a possible alternative treatment for selected patients. Adv Neurol (2004) 94:247–54.
102.
CandiaVSchäferTTaubERauHAltenmüllerERockstrohBet alSensory motor retuning: a behavioral treatment for focal hand dystonia of pianists and guitarists. Arch Phys Med Rehabil (2002) 83(10):1342–8. 10.1053/apmr.2002.35094
103.
PrioriAPesentiACappellariAScarlatoGBarbieriS. Limb immobilization for the treatment of focal occupational dystonia. Neurology (2001) 57(3):405–9. 10.1212/wnl.57.3.405
104.
CandiaVElbertTAltenmüllerERauHSchäferTTaubE. Constraint-induced movement therapy for focal hand dystonia in musicians. Lancet (1999) 9146. 10.1016/j.parkreldis.2011.09.017
105.
FerraraJStameyWStruttAMAdamORJankovicJ. Transcutaneous electrical stimulation (TENS) for psychogenic movement disorders. J Neuropsychiatry Clin Neurosci Spring (2011) 23(2):141–8. 10.1176/jnp.23.2.jnp141
106.
CandiaVWienbruchCElbertTRockstrohBRayW. Effective behavioral treatment of focal hand dystonia in musicians alters somatosensory cortical organization. Proc Natl Acad Sci U S A. (2003) 100(13):7942–6. 10.1073/pnas.1231193100
107.
TibbenMIvan WensenENijenhuisBZwerverJ. Is behavioural therapy a new treatment option for task-specific dystonia in athletes? A case series. Tremor Other Hyperkinet Mov (N Y) (2023) 13:16. 10.5334/tohm.737
108.
KoboriO. Cognitive behavioural formulation for focal dystonia in a student athlete: a case report. Behav Cogn Psychotherapy (2007) 35(2):245–9. 10.1017/s1352465806003171
109.
WieckAHarringtonRMarksIMarsdenC. Writer's cramp: a controlled trial of habit reversal treatment. Br J Psychiatry (1988) 153:111–5. 10.1192/bjp.153.1.111
110.
HsiehYDeshpandeS. A therapy-led, multidisciplinary programme for treatment-resistant functional fixed dystonia. BMJ Case Rep (2020) 13(7):e235213. 10.1136/bcr-2020-235213
111.
KhachaneYKozlowskaKSavageBMcClureGButlerGGrayNet alTwisted in pain: the multidisciplinary treatment approach to functional dystonia. Harv Rev Psychiatry (2019) 27(6):359–81. 10.1097/hrp.0000000000000237
112.
ChudleighCKozlowskaKKothurKDaviesFBaxterHLandiniAet alManaging non-epileptic seizures and psychogenic dystonia in an adolescent girl with preterm brain injury. Harv Rev Psychiatry (2013) 21(3):163–74. 10.1097/HRP.0b013e318293b29f
113.
ChatterjeeSSDasSGuptaSBhattacharyaS. The twisted mind - psychogenic dystonia in an adolescent, responding to antidepressant therapy. Shanghai Arch Psychiatry. (2018) 30(2):133–4. 10.11919/j.issn.1002-0829.217114
114.
Puccioni-SohlerMRamosJRosadasCVasconcellosLF. Psychogenic movement disorder in human T-lymphotropic virus type 1 associated myelopathy. Int J Infect Dis (2016) 42:47–9. 10.1016/j.ijid.2015.11.013
115.
Wagle ShuklaAVaillancourtDE. Treatment and physiology in parkinson's disease and dystonia: using transcranial magnetic stimulation to uncover the mechanisms of action. Curr Neurol Neurosci Rep (2014) 14(6):449. 10.1007/s11910-014-0449-5
116.
NielsenGStoneJMatthewsABrownMSparkesCFarmerRet alPhysiotherapy for functional motor disorders: a consensus recommendation. J Neurol Neurosurg Psychiatry (2015) 86(10):1113–9. 10.1136/jnnp-2014-309255
117.
PickSAndersonDGAsadi-PooyaAAAybekSBasletGBloemBRet alOutcome measurement in functional neurological disorder: a systematic review and recommendations. J Neurol Neurosurg Psychiatry (2020) 91(6):638–49. 10.1136/jnnp-2019-322180
118.
HallettM. Progress in physical therapy for functional motor disorder. Lancet Neurol (2024) 23(7):650–1. 10.1016/s1474-4422(24)00162-5
119.
TsuboiTCifLCoubesPOstremJLRomeroDAMiyagiYet alSecondary worsening following DYT1 dystonia deep brain stimulation: a multi-country cohort. Front Hum Neurosci (2020) 14:242. 10.3389/fnhum.2020.00242
Summary
Keywords
systematic review, rehabilitation, physical therapy, exercise therapy, immobilization
Citation
Kamo H, Nagaki K, Kraus AR, Warren L and Wagle Shukla A (2025) Neurorehabilitation in dystonia care: key questions of who benefits, what modalities, and when to intervene. Dystonia 4:14695. doi: 10.3389/dyst.2025.14695
Received
28 March 2025
Accepted
30 July 2025
Published
22 August 2025
Volume
4 - 2025
Edited by
Giovanni Battistella, Massachusetts Eye & Ear Infirmary and Harvard Medical School, United States
Updates
Copyright
© 2025 Kamo, Nagaki, Kraus, Warren and Wagle Shukla.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aparna Wagle Shukla, aparna.shukla@neurology.ufl.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.