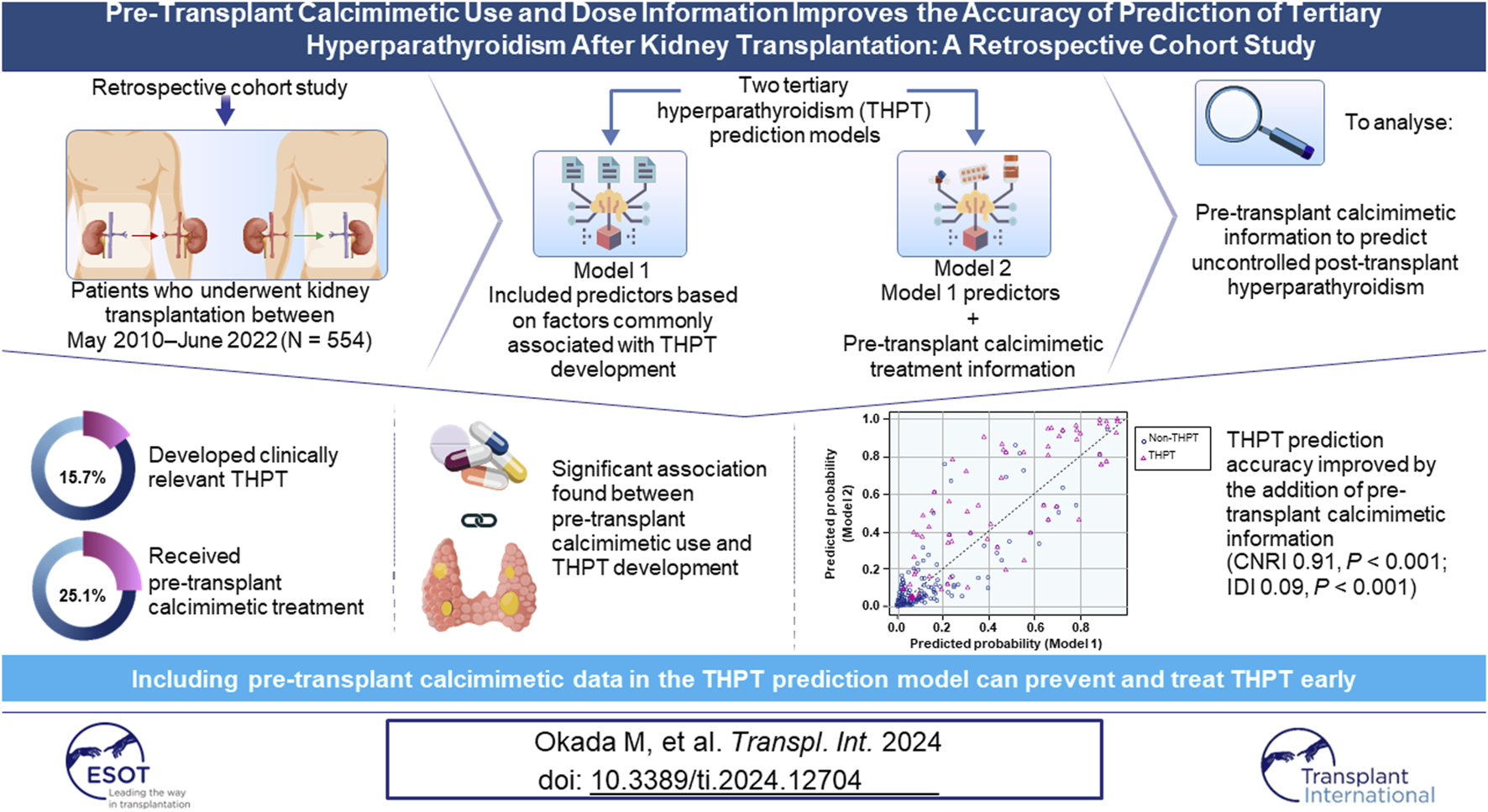
Abstract
Tertiary hyperparathyroidism (THPT) is characterized by elevated parathyroid hormone and serum calcium levels after kidney transplantation (KTx). To ascertain whether pre-transplant calcimimetic use and dose information would improve THPT prediction accuracy, this retrospective cohort study evaluated patients who underwent KTx between 2010 and 2022. The primary outcome was the development of clinically relevant THPT. Logistic regression analysis was used to evaluate pre-transplant calcimimetic use as a determinant of THPT development. Participants were categorized into four groups according to calcimimetic dose, developing two THPT prediction models (with or without calcimimetic information). Continuous net reclassification improvement (CNRI) and integrated discrimination improvement (IDI) were calculated to assess ability to reclassify the degree of THPT risk by adding pre-transplant calcimimetic information. Of the 554 patients, 87 (15.7%) developed THPT, whereas 139 (25.1%) received pre-transplant calcimimetic treatment. Multivariate logistic regression analysis revealed that pre-transplant calcimimetic use was significantly associated with THPT development. Pre-transplant calcimimetic information significantly improved the predicted probability accuracy of THPT (CNRI and IDI were 0.91 [p < 0.001], and 0.09 [p < 0.001], respectively). The THPT prediction model including pre-transplant calcimimetic information as a predictive factor can contribute to the prevention and early treatment of THPT in the era of calcimimetics.
Graphical Abstract
Introduction
Persistent hyperparathyroidism after kidney transplantation (KTx) is associated with unfavorable kidney graft and patient outcomes [1–3]. Tertiary hyperparathyroidism (THPT) is characterized by high parathyroid hormone (PTH) and serum calcium (Ca) levels, even in functioning kidney grafts [4], and often requires therapeutic intervention [5–8]. Common treatment options for THPT include parathyroidectomy (PTx) and calcimimetics [9–11]. However, in KTx patients, PTx can increase serum creatinine levels [12, 13], and the disadvantages of calcimimetics include being off-label in some regions, high medical costs [14], and an increased risk of urinary stones [15, 16]. For patients at high risk of THPT, pre-transplant PTx is appropriate [17, 18].
The predictive factors for THPT include pre-transplant serum Ca and PTH levels, dialysis duration, and parathyroid gland size [19, 20]. Prediction models using only three variables (serum Ca, PTH levels, and dialysis duration) have been shown to accurately predict the risk of THPT [21]. However, recently, pre-transplant calcimimetic administration has also been reported as an additional predictive factor for THPT [22, 23].
The effectiveness of calcimimetics in the treatment of secondary hyperparathyroidism (SHPT) is widely recognized. In vitamin D-resistant SHPT, cinacalcet effectively reduces PTH levels [24, 25]. Several studies have demonstrated that cinacalcet prevents cardiovascular events and patient mortality [26–28]. Following cinacalcet, new calcimimetics have been developed [29, 30], and with an increase in treatment options, the proportion of dialysis patients receiving calcimimetic treatment is likely to increase. In this era of calcimimetics, pre-transplant calcimimetic use and dose information may predict THPT progression after KTx.
THPT risk assessment is complicated by several factors. In patients treated with calcimimetics, the assessment of THPT risk can be challenging because of the drastic decrease in serum Ca and PTH levels [31, 32]. Cianciolo et al. [33] proposed evaluating the need for PTx in KTx candidates receiving calcimimetic treatment after ceasing treatment for 2–4 weeks. However, discontinuation of calcimimetic treatment leads to a rapid increase in PTH levels, which may cause hyperparathyroidism-related adverse events and complicate the optimal timing of KTx. Therefore, assessment of THPT risk without discontinuing calcimimetic treatment is safer. A need for highly accurate prediction of THPT risk arises; this can contribute to the prevention and early treatment of THPT in patients undergoing KTx. Accurate THPT prediction models that include calcimimetic dose information are therefore required.
Hence, in this retrospective study, we aimed to investigate whether the inclusion of calcimimetic use and dose information as predictive factors in a prediction model could improve THPT prediction accuracy.
Materials and Methods
Data Source
Consecutive patients who underwent KTx between May 2010 and June 2022 were included. The data were collected on 30 June 2023.
Participants
The exclusion criteria were as follows: 1) PTx before KTx, 2) end-stage kidney disease with an estimated glomerular filtration rate (eGFR) of less than 15 mL/min/1.73 m2 within a year after KTx, 3) denosumab treatment within a year after KTx, 4) missing data, and 5) preemptive KTx. Data on patient age, sex, body mass index, original disease, dialysis duration, serum Ca and intact PTH levels, kidney graft function, parathyroid gland size (the size of the parathyroid glands of recipients were routinely measured by ultrasound before KTx), ABO blood type incompatibility, positivity for donor-specific human leukocyte antigen antibodies, and PTx and calcimimetic treatment histories, were collected.
All procedures involving participants were approved by the Institutional Review Board (IRB) and performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The IRB waived the requirement to obtain informed consent because of the retrospective nature of the study. Details of the study and its outcomes are available on our institutional website. This study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Outcome
The primary outcome was the development of clinically relevant THPT, defined as the presence of both hypercalcemia (total serum Ca ≥10.5 mg/dL) and high PTH level (intact PTH >80 pg/mL) 1 year after KTx, based on the guidelines of the Japanese Society for Dialysis Therapy [6, 34]. In addition, post-transplant PTx or calcimimetic therapy to control severe hyperparathyroidism was included in the definition of THPT.
Measurements
Pre-transplant blood sample analyses were performed in all patients within 3 months before KTx. Serum Ca levels were measured using standard methods. Intact PTH levels were measured using the following second-generation immunoassays: an electrochemical luminescence immunoassay (SRL, Tokyo, Japan1, reference range 10–65 pg/mL) and an enzyme immunoassay (Tosoh, Tokyo, Japan2, reference range 9–80 pg/mL). For serum albumin levels <4.0 g/dL, all serum Ca levels were corrected [35]. The eGFR was evaluated using the creatinine equation provided by the Japanese Society of Nephrology and the Japanese Society for Pediatric Nephrology [36, 37].
Immunosuppression
Immunosuppressive regimens included calcineurin inhibitors (cyclosporine or tacrolimus), mycophenolic acids, mizoribine, everolimus, and glucocorticoids. Basiliximab was used as induction therapy. In addition, rituximab administration or splenectomy was used as induction therapy in anti-donor antibody-positive patients before KTx, except in those with low antibody titers.
Statistical Analysis
Pearson’s chi-squared test was used to analyze nominal variables, and the Mann–Whitney U test or Student’s t-test was used for continuous variables. The normality of the distribution of the data was assessed using the Shapiro–Wilk normality test and histogram (Supplementary Table S1; Supplementary Figure S1). Statistical significance was set at p < 0.05.
First, logistic regression analysis was performed to confirm that known predictive factors were associated with the development of THPT, even after adjusting for the patient background between the THPT and non-THPT groups. Then, two THPT prediction models were constructed using logistic regression, one with and one without pretransplant calcimimetic use and dose information (Model 1 and Model 2). Owing to the non-linear relationship between serum Ca, intact PTH, dialysis duration, parathyroid gland size, and THPT risk (Supplementary Figure S1), these variables were transformed into categorical variables by dividing them into four categories based on the number of cases. The information on pre-transplant calcimimetic treatment was also used to categorize participants into four groups according to the tertile of cinacalcet dose per unit of body weight (mg/kg). Based on previous studies, evocalcet (2.0 mg/day) and etelcalcetide (7.5 mg/week) dosages were considered equivalent to a cinacalcet dosage of 25.0 mg/day [38, 39].
To evaluate the effect of the inclusion of pre-transplant calcimimetic information as a predictive factor for THPT, the accuracy of Models 1 and 2 were compared. First, scatter plots of the predicted probabilities of Models 1 and 2 were created, then continuous net reclassification improvement (CNRI) and integrated discrimination improvement (IDI) were calculated to assess the ability to reclassify the degree of THPT risk by adding pretransplant calcimimetic information [40–42]. To identify the characteristics of THPT patients for whom the addition of the pre-transplant calcimimetic information significantly improved the predictive probability, we stratified THPT cases by a change in predictive probability of 0.1 and compared the characteristics. In addition, receiver operating characteristic (ROC) curves for the predicted THPT probabilities of each model were obtained, and the areas under the curve (AUCs) were compared for the two models using Delong’s test [43].
Internal Validation
Internal validation of the prediction models was performed using the bootstrap method [44]. By resampling with replacement, 1,000 pseudo-external datasets were created, and the ROC AUC was obtained. Overfitting was assessed using slope optimism, and calibration was performed.
Easy R (EZR) version 1.61 (The R Foundation for Statistical Computing) was used for the statistical analyses [45]. The calculations of CNRI and IDI, as well as the internal validation by the bootstrap method, were performed using the R package “rms” (version 6.7–0). Statistical significance was set at p < 0.05.
Results
Participant Characteristics
A total of 554 patients met the inclusion criteria (median observation period, 81 months [interquartile range {IQR}: 47–122 months]; Figure 1). Of the 554 patients, 87 (15.7%) developed THPT after KTx, whereas 139 (25.1%) received calcimimetic treatment before KTx (Table 1, Supplementary Table S2). More than 70% of patients had pre-transplant hyperparathyroidism (i-PTH >80 pg/mL) with or without pre-transplant calcimimetic treatment (Supplementary Table S3). Significant differences were observed between the THPT and non-THPT groups in terms of dialysis duration, living donor, parathyroid gland size, pre-transplant calcimimetic use, and serum Ca and intact PTH levels (Table 1). In addition, serum Ca and intact PTH levels 1 year after KTx also significantly differed between the two groups (Table 2). In the THPT group (n = 87), 43 (49.4%) received PTx, and 36 (41.4%) received calcimimetic treatment after KTx (Table 2). Most PTx were done within 2 years after KTx (the median interval from KTx to PTx was 10.0 months [IQR: 7–17 months]), and post-transplant calcimimetic treatment was initiated within 1 year after KTx in all cases (Table 2).
FIGURE 1
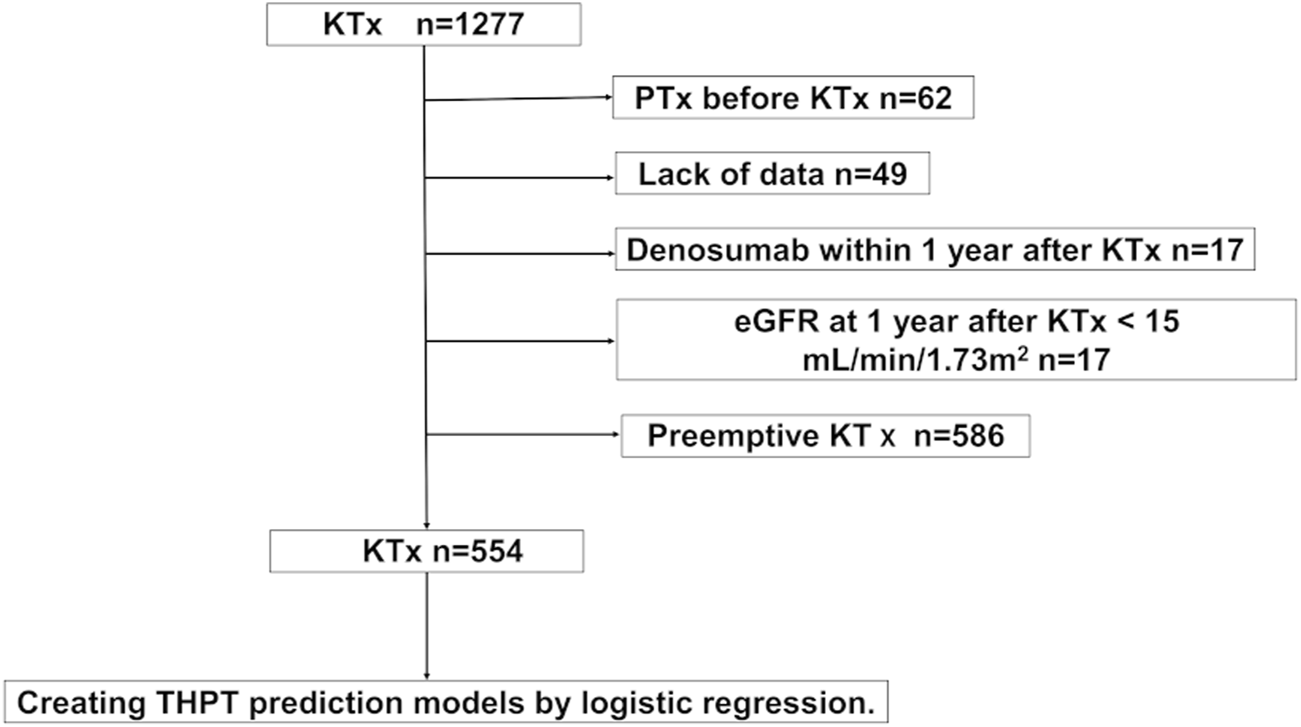
Participant selection flowchart. eGFR, estimated glomerular filtration rate; KTx, kidney transplantation; PTx, parathyroidectomy; THPT, tertiary hyperparathyroidism.
TABLE 1
| Total N = 554 | Non-THPT N = 467 | THPT N = 87 | p-value | |
|---|---|---|---|---|
| Recipient age (years, IQR) | 51 (39–62) | 50 (38–62) | 53 (46–62) | 0.060 |
| Recipient sex (male, %) | 352 (63.5) | 304 (65.1) | 48 (55.2) | 0.089 |
| Body mass index (kg/m2, SD) | 22.1 (3.7) | 22.1 (3.8) | 22.0 (3.3) | 0.807 |
| Dialysis vintage (months, IQR) | 21 (6–54) | 16 (5–38) | 112 (48–167) | <0.001* |
| Previous KTx (%) | 22 (4.0) | 18 (3.9) | 4 (4.6) | 0.764 |
| Living donor (%) | 506 (91.3) | 438 (93.8) | 68 (78.2) | <0.001* |
| Original disease (%) | 0.058 | |||
| Glomerular disease | 192 (34.7) | 159 (34.0) | 33 (37.9) | |
| Diabetic kidney disease | 141 (25.6) | 122 (26.1) | 19 (21.8) | |
| Polycystic kidney disease | 28 (5.1) | 19 (4.1) | 9 (10.3) | |
| Hypertensive kidney disease | 38 (6.9) | 36 (7.7) | 2 (2.3) | |
| Others | 49 (8.8) | 39 (8.4) | 10 (11.5) | |
| Unknown | 106 (19.1) | 92 (19.7) | 14 (16.1) | |
| Preformed DSA (%) | 40 (7.2) | 38 (8.1) | 2 (2.3) | 0.068 |
| ABO blood type incompatible kidney transplantation (%) | 160 (28.9) | 128 (27.4) | 32 (36.8) | 0.093 |
| Parathyroid gland size (mm, IQR) | 7.2 (5.1–9.8) | 6.3 (4.7–8.4) | 9.4 (7.1–11.6) | <0.001* |
| VDRA before KTx (%) | 352 (63.5) | 288 (61.7) | 64 (73.5) | 0.039* |
| Alfacalcidol | 184 (33.2) | 164 (35.1) | 20 (23.0) | |
| Calcitriol | 64 (11.5) | 47 (10.1) | 17 (19.5) | |
| Maxacalcitol | 104 (18.8) | 77 (16.5) | 27 (31.0) | |
| Calcimimetics before KTx (%) | 139 (25.1) | 84 (18.0) | 55 (63.2) | <0.001* |
| Cinacalcet | 89 (16.1) | 50 (10.7) | 39 (44.8) | |
| Evocalcet | 36 (6.5) | 25 (5.4) | 11 (12.6) | |
| Etelcalcetide | 14 (2.5) | 9 (1.9) | 5 (2.7) | |
| Calcimimetic dose per unit of body weight (mg/kg, IQR) | 0.4 (0.3–0.7) | 0.4 (0.3–0.5) | 0.6 (0.4–1.0) | <0.001* |
| Lab data before KTx | ||||
| Corrected calcium (mg/dL, IQR) | 9.3 (8.9–9.8) | 9.2 (8.9–9.7) | 9.8 (9.3–10.3) | <0.001* |
| Intact PTH (pg/mL, IQR) | 157.5 (85.0–248.0) | 145.0 (78.0–240.0) | 203 (154.5–317.5) | <0.001* |
Patient characteristics before KTx.
DSA, donor-specific HLA antibody; eGFR, estimated glomerular filtration rate; IQR, interquartile range; KTx, kidney transplantation; PTH, parathyroid hormone; SD, standard deviation; THPT, tertiary hyperparathyroidism; VDRA, vitamin D receptor activator.
The results of parathyroid gland size excluded patients in whom parathyroid gland was not detected by echography.
Calcimimetic dose was converted into cinacalcet dose and calculated by per unit of body weight, excluding patients who had not received pre-KTx calcimimetic treatment.
*p-value <0.05.
TABLE 2
| Total N = 554 | Non-THPT N = 467 | THPT N = 87 | p-value | |
|---|---|---|---|---|
| Lab data 1 year post-KTx | ||||
| Corrected calcium (mg/dL, IQR) | 9.7 (9.4–10.0) | 9.7 (9.4–9.9) | 10.6 (9.8–10.8) | <0.001* |
| Intact PTH (pg/mL, IQR) | 91.0 (65.0–130.0) | 86.0 (64.2–115.0) | 137.0 (88.9–181.0) | <0.001* |
| Recipient eGFR (mL/min/1.73 m2, IQR) | 44.2 (36.9–51.8) | 43.1 (36.4–51.2) | 44.2 (36.5–52.1) | 0.695 |
| Parathyroidectomy after KTx (%) | 43 (4.0) | 0 (0.0) | 43 (49.4) | <0.001* |
| Interval between KTx and PTx | NA | |||
| <=12 months | NA | NA | 25 (58.1%) | |
| 13–24 months | NA | NA | 14 (32.6) | |
| >24 months | NA | NA | 4 (9.3) | |
| Calcimimetics after KTx (%) | 36 (3.1) | 0 (0.0) | 36 (41.4) | <0.001* |
| Follow up after KTx (months, IQR) | 81 (47–122) | 81 (47–122) | 89 (55–119) | 0.371 |
Clinical data after KTx.
eGFR, estimated glomerular filtration rate; IQR, interquartile range; KTx, kidney transplantation; NA, not applicable; PTH, parathyroid hormone; PTx, parathyroidectomy; THPT, tertiary hyperparathyroidism.
*p-value <0.05.
THPT Predictive Factors
Multivariate logistic regression analysis of predictive factors for THPT development revealed that dialysis duration, pre-transplant serum Ca levels, intact PTH levels, parathyroid gland size, and pre-transplant calcimimetic use were significantly associated with THPT (Table 3).
TABLE 3
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Factors | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Living donor | 0.24 | 0.13–0.45 | <0.001* | 0.73 | 0.25–2.14 | 0.568 |
| Preformed DSA | 0.27 | 0.06–1.12 | 0.071 | 0.12 | 0.01–1.48 | 0.098 |
| Pretransplant VDRA use | 1.73 | 1.04–2.88 | 0.036* | 1.90 | 0.87–4.16 | 0.109 |
| Dialysis duration (months, reference to <6) | ||||||
| 6–20 | 0.75 | 0.24–2.28 | 0.609 | 0.88 | 0.24–3.22 | 0.841 |
| 21–53 | 1.50 | 0.56–3.99 | 0.419 | 0.62 | 0.18–2.18 | 0.457 |
| 54– | 14.30 | 6.21–32.70 | <0.001* | 6.99 | 2.26–21.70 | <0.001* |
| Serum Ca before KTx (mg/dL, reference to <8.9) | ||||||
| 8.9–9.2 | 0.76 | 0.29–2.00 | 0.581 | 1.39 | 0.37–5.21 | 0.627 |
| 9.3–9.7 | 2.67 | 1.23–5.77 | 0.013* | 4.58 | 1.51–13.90 | 0.007* |
| 9.8– | 5.35 | 2.56–11.20 | <0.001* | 16.90 | 5.16–55.20 | <0.001* |
| Intact PTH before KTx (pg/mL, reference to <85.0) | ||||||
| 85.0–157.0 | 3.27 | 1.26–8.52 | 0.015* | 11.50 | 2.96–44.70 | <0.001* |
| 158.0–247.0 | 6.29 | 2.52–15.70 | <0.001* | 19.30 | 5.38–69.30 | <0.001* |
| 248.0– | 6.66 | 2.69–16.50 | <0.001* | 28.50 | 7.65–106.00 | <0.001* |
| Parathyroid gland size before KTx (mm, reference to 0) | ||||||
| 0.1–5.7 | 2.10 | 0.90–4.86 | 0.085 | 1.34 | 0.45–3.99 | 0.602 |
| 5.8–8.8 | 4.79 | 2.40–9.57 | <0.001* | 3.53 | 1.32–9.44 | 0.012* |
| 8.9– | 17.60 | 9.27–33.40 | <0.001* | 12.30 | 4.46–34.00 | <0.001* |
| Pretransplant calcimimetics use | 7.84 | 4.77–12.90 | <0.001* | 10.80 | 4.73–24.60 | <0.001* |
Logistic regression for THPT development.
Ca, Calcium; 95% CI, 95% confidence interval; DSA, donor-specific HLA antibody; KTx, kidney transplantation; OR, odds ratio; PTH, parathyroid hormone; THPT, tertiary hyperparathyroidism; VDRA, vitamin D receptor activator.
The parathyroid gland size was defined as 0 when parathyroid gland was not detected by echography.
*p-value <0.05.
THPT Prediction Models
Two THPT prediction models were created based on the logistic regression analysis. Model 1 was created from four predictors: dialysis duration, serum Ca level, intact PTH level, and parathyroid gland size, whereas Model 2 was created by adding the calcimimetic dose per unit of body weight to the predictors used in Model 1 (Table 4).
TABLE 4
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Variable | RC (SE) | OR (95% CI) | p-value | RC (SE) | OR (95% CI) | p-value |
| (Intercept) | −6.26 (0.79) | −7.57 (0.94) | ||||
| Dialysis duration (months, reference to < 6) | ||||||
| 6–20 | −0.07 (0.62) | 0.94 (0.28–3.13) | 0.913 | −0.19 (0.67) | 0.83 (0.87–3.05) | 0.775 |
| 21–53 | 0.11 (0.57) | 1.11 (0.36–3.41) | 0.852 | −0.52 (0.65) | 0.59 (0.17–2.13) | 0.423 |
| 54– | 2.40 (0.50) | 11.0 (4.12–29.60) | <0.001 | 1.84 (0.56) | 6.27 (2.10–18.70) | 0.001 |
| Serum Ca (mg/dL, reference to < 8.9) | ||||||
| 8.9–9.2 | −0.42 (0.58) | 0.66 (0.21–2.06) | 0.470 | 0.23 (0.68) | 1.26 (0.33–4.80) | 0.736 |
| 9.3–9.7 | 1.07 (0.57) | 2.91 (1.11–7.58) | 0.029 | 1.43 (0.56) | 4.18 (1.38–12.60) | 0.011 |
| 9.8– | 1.82 (0.50) | 6.20 (2.33–16.50) | <0.001 | 2.70 (0.59) | 15.00 (4.72–47.40) | <0.001 |
| Intact PTH (pg/mL, reference to < 85.0) | ||||||
| 85.0–157.0 | 1.55 (0.58) | 4.71 (1.51–14.70) | 0.008 | 2.27 (0.66) | 9.69 (2.65–35.40) | 0.001 |
| 158.0–247.0 | 2.70 (0.58) | 14.90 (4.80–46.50) | <0.001 | 2.85 (0.63) | 17.40 (5.00–60.20) | <0.001 |
| 248.0– | 2.63 (0.58) | 13.8 (4.44–43.20) | <0.001 | 3.17 (0.64) | 23.80 (6.73–83.90) | <0.001 |
| Parathyroid gland size (mm, reference to 0) | ||||||
| 0.1–5.7 | 0.83 (0.50) | 2.29 (0.86–6.08) | 0.096 | 0.30 (0.55) | 1.35 (0.46–3.97) | 0.579 |
| 5.8–8.8 | 1.45 (0.46) | 4.27 (1.74–10.50) | 0.002 | 1.28 (0.49) | 3.61 (1.37–9.50) | 0.009 |
| 8.9– | 2.54 (0.44) | 12.60 (5.31–30.00) | <0.001 | 2.33 (0.53) | 10.20 (3.65–28.80) | <0.001 |
| Calcimimetic dose per unit of body weight (mg/kg, reference to 0) | ||||||
| 0.1–0.2 | NA | NA | NA | 1.88 (0.60) | 6.54 (2.04–21.00) | 0.002 |
| 0.3–0.4 | NA | NA | NA | 2.23 (0.58) | 9.32 (3.02–28.80) | <0.001 |
| 0.5– | NA | NA | NA | 2.95 (0.55) | 19.10 (6.55–55.70) | <0.001 |
Logistic regression THPT prediction models.
Ca, calcium; 95% CI, 95% confidence interval; NA, not applicable; OR, odds ratio; PTH, parathyroid hormone; RC, regression coefficient; SE, standard error.
The parathyroid gland size was defined as 0 when parathyroid gland was not detected by echography.
Calcimimetic dose was converted into cinacalcet dose and calculated by per unit of body weight and is only adopted as a predictive factor in Model 2.
Effect of the Pre-Transplant Calcimimetic Information on THPT Prediction
Figure 2 shows scatter plots of the predicted probabilities of Models 1 and 2. When comparing the predicted probabilities of the two THPT prediction models, the addition of the pre-transplant calcimimetic information improved the predicted probabilities in 65.5% (57/87) of the THPT group and 80.1% (374/467) of the non-THPT group, respectively (Figure 2; Table 5). The CNRI calculated from the sum of the proportion of improvement/worsening of the predicted probabilities was 0.91 (95% CI: 0.70–1.13, p < 0.001) (Figure 2; Table 5). In contrast, the mean changes in predicted probabilities were 0.08 in the THPT group and 0.01 in the non-THPT group, resulting in an IDI of 0.09 (95% CI: 0.05–0.13, p < 0.001) (Figure 2; Table 5). In the subgroup of THPT with an improvement of 0.1 or more in predictive probabilities by adding the pre-transplant calcimimetic information, both the proportion of patients receiving pretransplant calcimimetics and the doses of pre-transplant calcimimetics were significantly higher (Table 6).
FIGURE 2
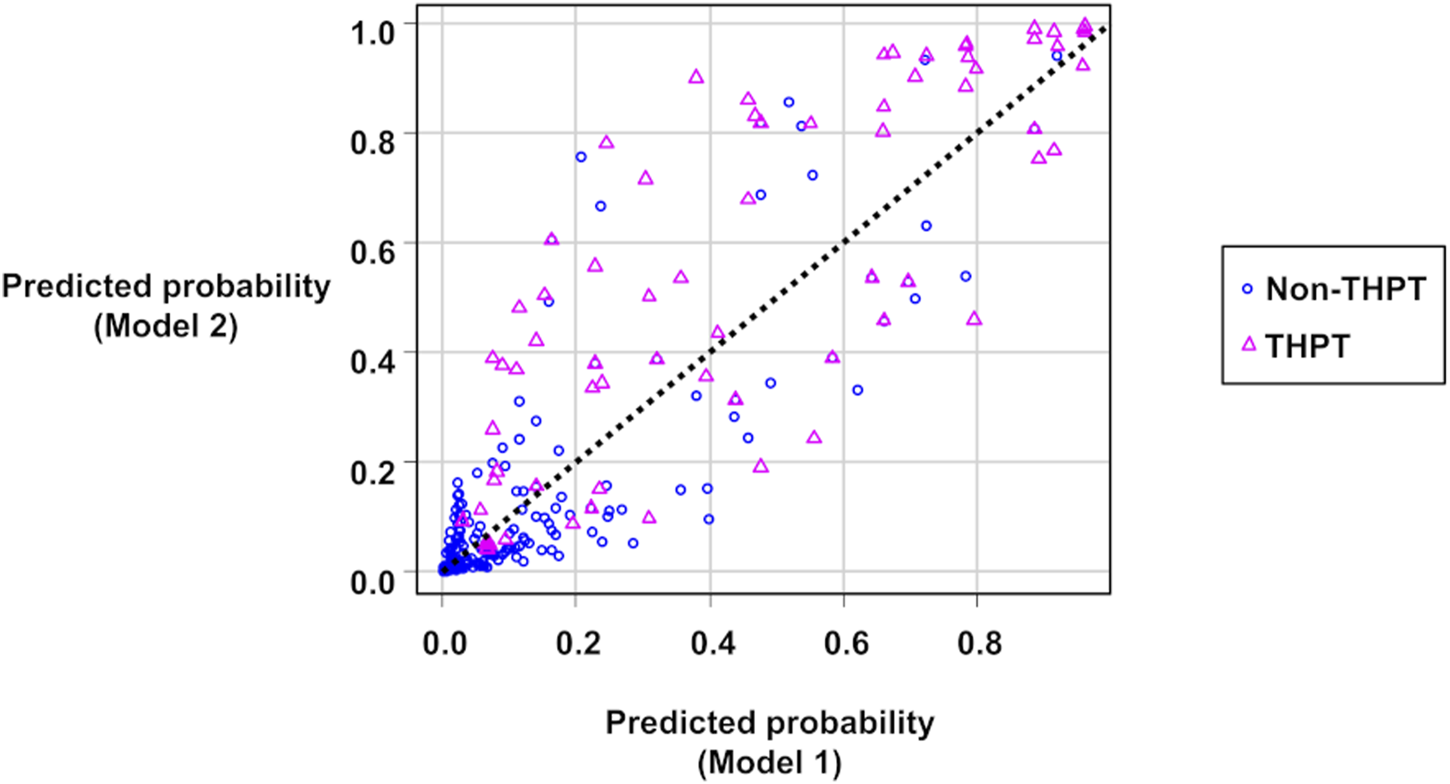
Scatter plots of the predicted probabilities of Model 1 and Model 2. The circles represent non-THPT cases, and the triangles represent THPT cases. The black dashed line represents the coordinates where the predictions of Model 1 and Model 2 match. The circles below the black dashed line or the triangles above it indicate that the THPT predictions have improved in Model 2 compared with Model 1. THPT, tertiary hyperparathyroidism.
TABLE 5
| Proportions of positive and negative changes in predicted probabilities | ||||
| (1) Increase of predicted probability for THPT group: 0.655 (57/87) | ||||
| (2) Increase of predicted probability for non-THPT group: 0.199 (93/467) | ||||
| (3) Decrease of predicted probability for THPT group: 0.345 (30/87) | ||||
| (4) Decrease of predicted probability for non-THPT group: 0.801 (374/467) | ||||
| CNRI | Index (SE) | Z value | p-value | 95% CI |
| CNRI for THPT group (1–3) | 0.31 (0.10) | 3.05 | 0.002* | 0.11–0.51 |
| CNRI for non-THPT group (4–2) | 0.60 (0.04) | 16.28 | <0.001* | 0.53–0.67 |
| CNRI for entire cohort (1–3+4–2) | 0.91 (0.11) | 8.4 | < 0.001* | 0.70–1.13 |
| Mean change in predicted probability | ||||
| Increase for THPT group (sensitivity): 0.08 | ||||
| Decrease for non-THPT group (specificity): 0.01 | ||||
| IDI | Index (SE) | Z value | p-value | 95% CI |
| 0.09 (0.02) | 4.35 | <0.001* | 0.05–0.13 | |
Summary of the calculation for CNRI and IDI for Model 2 compared to Model 1.
95% CI, 95% confidential interval; CNRI, continuous net reclassification improvement; IDI, integrated discrimination improvement; THPT, tertiary hyperparathyroidism; SE, standard error.
*p-value <0.05.
The bold values represent the final results of the analysis.
TABLE 6
| PP improvement <0.1 n = 48 | PP improvement >=0.1 n = 39 | p-value | |
|---|---|---|---|
| Dialysis duration (months, IQR) | 95 (45–146) | 123 (67–171) | 0.294 |
| Serum Ca before KTx (mg/dL, IQR) | 9.9 (9.50–10.4) | 9.6 (9.0–10.0) | 0.059 |
| Serum intact PTH before KTx (pg/mL, IQR) | 239.5 (177.3–341.8) | 190.0 (122.0–286.5) | 0.067 |
| Parathyroid gland size (mm, IQR) | 9.0 (0.0–11.0) | 5.5 (0.0–8.80) | 0.05 |
| Pre-transplant calcimimetic treatment (%) | 16 (33.3) | 39 (100.0) | <0.001* |
| Pre-transplant calcimimetic dose per unit of body weight (mg/kg, IQR) | 0.0 (0.0–0.3) | 0.7 (0.4–1.1) | <0.001* |
Characteristics of THPT patients classified by degree of improvement in predicted probability.
Ca, calcium; IQR, interquartile range; KTx, kidney transplantation; PTH, parathyroid hormone; PP, predicted probability; THPT, tertiary hyperparathyroidism.
Calcimimetic dose was converted into cinacalcet dose and calculated by per unit of body weight.
*p-value <0.05.
When comparing the ROC AUCs of the two THPT prediction models, the inclusion of the pretransplant calcimimetic information significantly improved the AUC from 0.92 (95% CI: 0.90–0.95, cut-off value: 0.20, specificity: 0.89, sensitivity: 0.79) to 0.95 (95% CI: 0.93–0.97, cut off value: 0.15, specificity: 0.89, sensitivity: 0.89) (p < 0.001) (Figure 3; Supplementary Table S4).
FIGURE 3
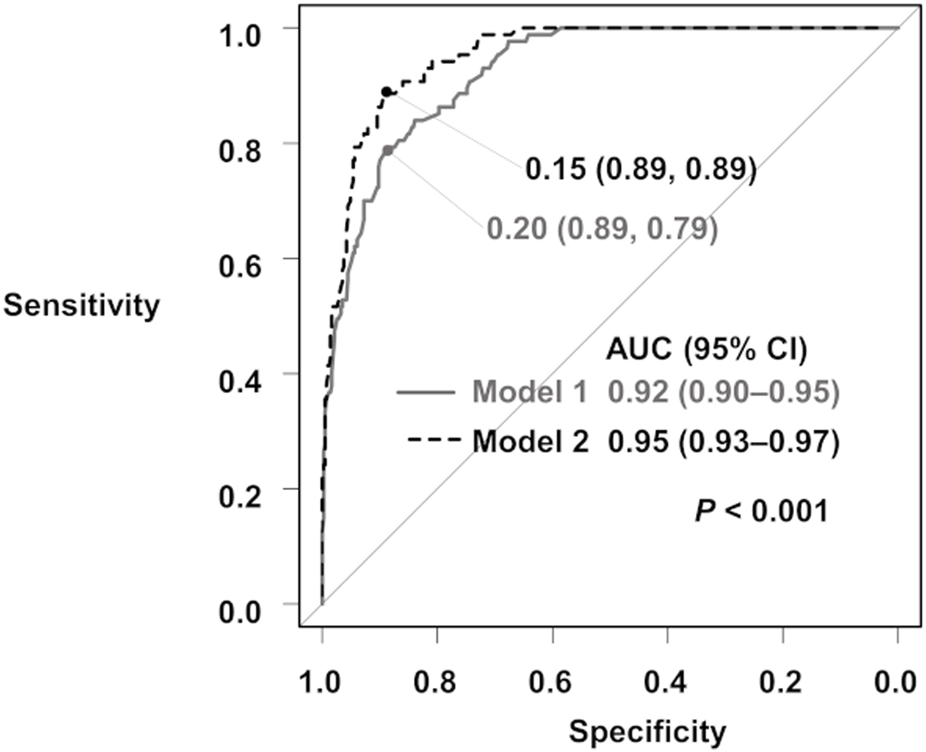
ROC curves for the prediction of THPT from Model 1 and Model 2. The gray curve is the ROC curve for Model 1, and the black dashed curve is the ROC curve for Model 2. The ROC AUCs and 95% CIs are shown. AUC, area under the curve; 95% CI, 95% confidence interval; ROC, receiver operating characteristic; THPT, tertiary hyperparathyroidism.
Internal Validation of THPT Prediction Models
The bootstrapped ROC AUCs for Models 1 and 2 were 0.91 and 0.94, respectively (Table 7). The slope optimism values of the two models were 0.11 and 0.16, respectively (Table 7). From the calibration diagrams based on the bootstrap validation results, although Model 1 outperformed Model 2 in the 0.3–0.5 probability range, Model 2 outperformed Model 1 in the 0.5–0.8 probability range. Both prediction models slightly underestimated THPT risk at low-risk levels and slightly overestimated it at high-risk levels (Figure 4).
TABLE 7
| Model 1 | Model 2 | |
|---|---|---|
| ROC AUC obtained through bootstrap resampling | 0.91 | 0.94 |
| Slope (BOC) | 0.11 | 0.16 |
| Mean absolute error | 0.03 | 0.03 |
| Mean squared error | 0.00 | 0.00 |
| 0.9 Quantile of absolute error | 0.06 | 0.08 |
Internal validation using the bootstrap method for the THPT prediction models.
BOC, bootstrap optimism corrected; ROC AUC, receiver operating characteristic area under the curve.
FIGURE 4
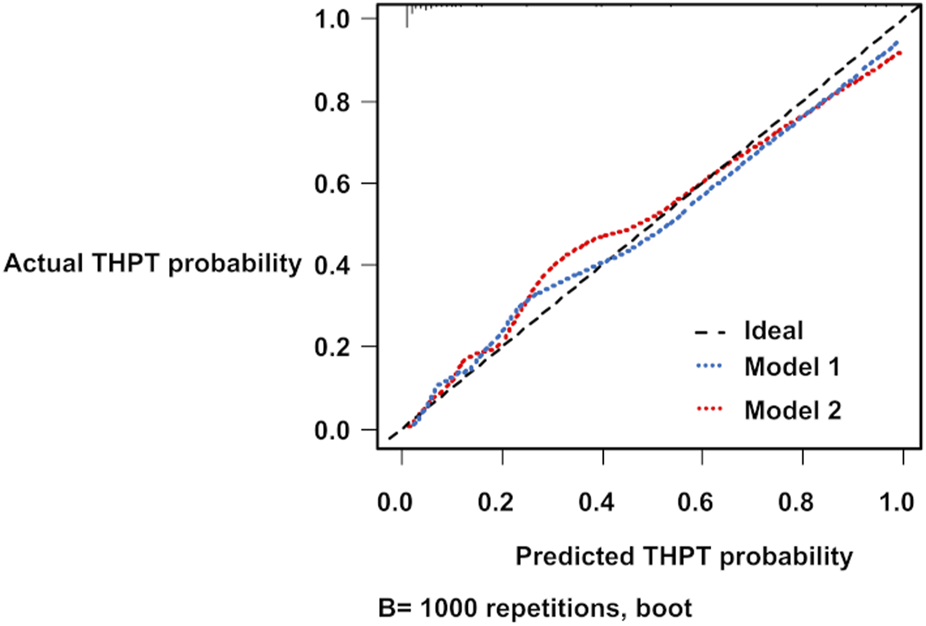
Calibration diagrams for THPT prediction models using the bootstrap method. The blue and red dashed lines represent the calibration diagrams for Model 1 and Model 2, respectively. THPT, tertiary hyperparathyroidism.
Discussion
THPT is a complication often observed after KTx, and post-transplant PTx or calcimimetic induction is often necessary [10, 11]. In this study, including the pre-transplant calcimimetic use and dose information as a predictive factor improved the accuracy of THPT prediction. From the scatter plot of the predicted probabilities of Model 1 and Model 2, the addition of pre-transplant calcimimetic information enhanced the accuracy of prediction of THPT risk in most cases in both the THPT and non-THPT groups, leading to high CNRI values. However, although the ROC AUC of Model 2 was significantly better than that of Model 1, the degree of improvement was relatively modest, contrary to the high CNRI value. In other words, Model 1 was able to predict THPT reasonably well even without pre-transplant calcimimetic information. This is probably because the proportion of patients who had received pre-transplant calcimimetic treatment was not as high, at 25% of the entire cohort. However, the subgroup analysis showed that patients treated with pre-transplant calcimimetics and at higher doses had greatly improved predictive probability. Thus, the larger the proportion of patients receiving pre-transplant calcimimetics and the calcimimetic dose in a cohort, the greater the contribution of calcimimetic information to THPT prediction improvement.
From the kidney graft function and prognosis perspective, pre-transplant PTx may be considered for cases with high THPT risk. For pre-transplant PTx to be properly performed, accurate THPT prediction is indispensable; however, research on THPT prediction models remains limited. Hong et al. [21] developed an excellent predictive model for THPT based on Ca, PTH, and dialysis duration. That study was a pioneering one on THPT prediction and holds significant importance for the prevention and early treatment of THPT. Yet, in that report, there was no mention of a relationship between calcimimetic use and THPT risk. In Japan, since the introduction of cinacalcet in 2008, the number of PTx in dialysis patients has drastically decreased [46]; however, the proportion of post-transplant hyperparathyroidism has not seen a corresponding decrease [3]. Calcimimetics are highly effective against SHPT; however, significant reductions in both PTH and calcium levels may lead to consequent underestimation of THPT risk for patients who should ideally undergo pre-transplant PTx. Therefore, in regions where calcimimetics are widely used, there is a potential risk of misestimating THPT risk.
To the best of our knowledge, this study represents the first report to validate a THPT prediction model that includes pre-transplant use and dose information of calcimimetics. By incorporating pre-transplant calcimimetic information into the predictive model, it becomes possible to properly assign high-THPT risk cases with suppressed PTH and Ca levels under calcimimetic treatment to the high-risk group. This contributes to pre-transplant PTx decision-making without discontinuing calcimimetics. In the context of widespread calcimimetic treatment, information on calcimimetic use and dose would be important for accurate THPT risk prediction.
As THPT prediction advances, candidates for pre-transplant PTx may be identified more frequently. However, the validity of postponing already scheduled KTx for the purpose of pre-transplant PTx remains uncertain. This is because the extension of dialysis duration is associated with poor patient and graft outcomes [47, 48]. The lack of evidence on whether the benefits of pre-transplant PTx outweigh those of shorter dialysis duration is a factor in this uncertainty. Therefore, the timing of PTx should be carefully considered on a case-by-case basis.
This study had some limitations. First, this was a single-center, retrospective study. Second, serum phosphorus data were lacking to evaluate its clinical relevance as a key factor influencing PTH levels [49]. Third, assessment of parathyroid gland size is another challenge as noted in a previous study [50]. There is a certain concern in reproducibility of ultrasound-guided parathyroid gland size measurement. Fourth, the prediction models were not externally validated. Fifth, our cohort was predominantly composed of patients receiving KTx from living donors, a scenario unique to Japan and distinct from Western countries. In addition, the prevalence of calcimimetic use and dialysis practices may differ between countries. Therefore, the prediction models used in this study may not be effective in predicting THPT in KTx candidates from other countries. However, the strengths of this study include the simplicity of the development methods for the prediction models and the use of analytical techniques with free statistical software. Thus, replicating the methods of this study in various cohorts from different regions using patient data would enable the convenient and cost-effective creation of an accurate predictive model.
In conclusion, information on pre-transplant calcimimetic use and dose improved the accuracy of post-KTx THPT prediction. The THPT prediction model that included pre-transplant calcimimetic use and dose information as a predictive factor can contribute to the prevention and early treatment of THPT in the era of calcimimetics. Future studies should perform external validations using new cohorts or cohorts from other institutions.
Statements
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving humans were approved by the Institutional Review Board of the Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective nature of the study.
Author Contributions
MO: conceptualization, methodology, writing–original draft. TS: writing–original draft. TaH: conceptualization, methodology, writing–review and editing. ToH, YH, KF, TI, and NG: investigation, formal analysis, visualization. SN: writing–review and editing. YW: supervision, writing–review and editing. Each author has reviewed the manuscript, believes it represents valid work, and approves it for submission. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank Editage (www.editage.com) for the English language editing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.12704/full#supplementary-material
References
1
Pihlstrøm H Dahle DO Mjøen G Pilz S März W Abedini S et al Increased Risk of All-Cause Mortality and Renal Graft Loss in Stable Renal Transplant Recipients with Hyperparathyroidism. Transplantation (2015) 99(2):351–9. 10.1097/TP.0000000000000583
2
Araujo M Ramalho JAM Elias RM Jorgetti V Nahas W Custodio M et al Persistent Hyperparathyroidism as a Risk Factor for Long-Term Graft Failure: The Need to Discuss Indication for Parathyroidectomy. Surgery (2018) 163(5):1144–50. 10.1016/j.surg.2017.12.010
3
Okada M Tominaga Y Sato T Tomosugi T Futamura K Hiramitsu T et al Elevated Parathyroid Hormone One Year after Kidney Transplantation Is an Independent Risk Factor for Graft Loss Even without Hypercalcemia. BMC Nephrol (2022) 23(1):212. 10.1186/s12882-022-02840-5
4
Fraser WD . Hyperparathyroidism. Lancet. (2009) 374(9684):145–58. 10.1016/S0140-6736(09)60507-9
5
Tang JA Friedman J Hwang MS Salapatas AM Bonzelaar LB Friedman M . Parathyroidectomy for Tertiary Hyperparathyroidism: A Systematic Review. Am J Otolaryngol (2017) 38(5):630–5. 10.1016/j.amjoto.2017.06.009
6
Fukagawa M Yokoyama K Koiwa F Taniguchi M Shoji T Kazama JJ et al Clinical Practice Guideline for the Management of Chronic Kidney Disease-Mineral and Bone Disorder. Ther Apher Dial (2013) 17(3):247–88. 10.1111/1744-9987.12058
7
Egbuna OI Taylor JG Bushinsky DA Zand MS . Elevated Calcium Phosphate Product after Renal Transplantation Is a Risk Factor for Graft Failure. Clin Transpl (2007) 21(4):558–66. 10.1111/j.1399-0012.2007.00690.x
8
Moore J Tomson CR Tessa Savage M Borrows R Ferro CJ . Serum Phosphate and Calcium Concentrations Are Associated with Reduced Patient Survival Following Kidney Transplantation. Clin Transpl (2011) 25(3):406–16. 10.1111/j.1399-0012.2010.01292.x
9
Dulfer RR Franssen GJH Hesselink DA Hoorn EJ van Eijck CHJ van Ginhoven TM . Systematic Review of Surgical and Medical Treatment for Tertiary Hyperparathyroidism. Br J Surg (2017) 104(7):804–13. 10.1002/bjs.10554
10
Cruzado JM Moreno P Torregrosa JV Taco O Mast R Gómez-Vaquero C et al A Randomized Study Comparing Parathyroidectomy with Cinacalcet for Treating Hypercalcemia in Kidney Allograft Recipients with Hyperparathyroidism. J Am Soc Nephrol (2016) 27(8):2487–94. 10.1681/ASN.2015060622
11
Finnerty BM Chan TW Jones G Khader T Moore M Gray KD et al Parathyroidectomy versus Cinacalcet in the Management of Tertiary Hyperparathyroidism: Surgery Improves Renal Transplant Allograft Survival. Surgery (2019) 165(1):129–34. 10.1016/j.surg.2018.04.090
12
Schwarz A Rustien G Merkel S Radermacher J Haller H . Decreased Renal Transplant Function after Parathyroidectomy. Nephrol Dial Transpl (2007) 22(2):584–91. 10.1093/ndt/gfl583
13
Parikh S Nagaraja H Agarwal A Samavedi S Von Visger J Nori U et al Impact of post-Kidney Transplant Parathyroidectomy on Allograft Function. Clin Transpl (2013) 27(3):397–402. 10.1111/ctr.12099
14
Schneider R Kolios G Koch BM Fernández ED Bartsch DK Schlosser K . An Economic Comparison of Surgical and Medical Therapy in Patients with Secondary Hyperparathyroidism--The German Perspective. Surgery (2010) 148(6):1091–9. 10.1016/j.surg.2010.09.009
15
Borchhardt KA Heinzl H Mayerwöger E Hörl WH Haas M Sunder-Plassmann G . Cinacalcet Increases Calcium Excretion in Hypercalcemic Hyperparathyroidism after Kidney Transplantation. Transplantation (2008) 86(7):919–24. 10.1097/TP.0b013e318186b7fb
16
Seager CM Srinivas TR Flechner SM . Development of Nephrolithiasis in a Renal Transplant Patient during Treatment with Cinacalcet. Ann Transpl (2013) 18:31–5. 10.12659/AOT.883809
17
Littbarski SA Kaltenborn A Gwiasda J Beneke J Arelin V Schwager Y et al Timing of Parathyroidectomy in Kidney Transplant Candidates with Secondary Hyperparathryroidism: Effect of Pretransplant versus Early or Late post-Transplant Parathyroidectomy. Surgery (2018) 163(2):373–80. 10.1016/j.surg.2017.10.016
18
Okada M Hiramitsu T Ichimori T Goto N Narumi S Watarai Y et al Comparison of Pre and Post-Transplant Parathyroidectomy in Renal Transplant Recipients and the Impact of Parathyroidectomy Timing on Calcium Metabolism and Renal Allograft Function: A Retrospective Single-Center Analysis. World J Surg (2020) 44(2):498–507. 10.1007/s00268-019-05124-6
19
Yamamoto T Tominaga Y Okada M Hiramitsu T Tsujita M Goto N et al Characteristics of Persistent Hyperparathyroidism after Renal Transplantation. World J Surg (2016) 40(3):600–6. 10.1007/s00268-015-3314-z
20
Kirnap NG Kirnap M Sayin B Akdur A Bascil Tutuncu N Haberal M . Risk Factors and Treatment Options for Persistent Hyperparathyroidism after Kidney Transplantation. Transpl Proc (2020) 52(1):157–61. 10.1016/j.transproceed.2019.11.020
21
Hong N Lee J Kim HW Jeong JJ Huh KH Rhee Y . Machine Learning-Derived Integer-Based Score and Prediction of Tertiary Hyperparathyroidism Among Kidney Transplant Recipients: An Integer-Based Score to Predict Tertiary Hyperparathyroidism. Clin J Am Soc Nephrol (2022) 17(7):1026–35. 10.2215/CJN.15921221
22
Sutton W Chen X Patel P Karzai S Prescott JD Segev DL et al Prevalence and Risk Factors for Tertiary Hyperparathyroidism in Kidney Transplant Recipients. Surgery (2022) 171(1):69–76. 10.1016/j.surg.2021.03.067
23
Walkenhorst Z Maskin A Westphal S Fingeret AL . Factors Associated with Persistent Post-Transplant Hyperparathyroidism after Index Renal Transplantation. J Surg Res (2023) 285:229–35. 10.1016/j.jss.2022.12.030
24
Goodman WG Hladik GA Turner SA Blaisdell PW Goodkin DA Liu W et al The Calcimimetic Agent AMG 073 Lowers Plasma Parathyroid Hormone Levels in Hemodialysis Patients with Secondary Hyperparathyroidism. J Am Soc Nephrol (2002) 13(4):1017–24. 10.1681/ASN.V1341017
25
Quarles LD Sherrard DJ Adler S Rosansky SJ McCary LC Liu W et al The Calcimimetic AMG 073 as a Potential Treatment for Secondary Hyperparathyroidism of End-Stage Renal Disease. J Am Soc Nephrol (2003) 14(3):575–83. 10.1097/01.asn.0000050224.03126.ad
26
Cunningham J Danese M Olson K Klassen P Chertow GM . Effects of the Calcimimetic Cinacalcet HCl on Cardiovascular Disease, Fracture, and Health-Related Quality of Life in Secondary Hyperparathyroidism. Kidney Int (2005) 68(4):1793–800. 10.1111/j.1523-1755.2005.00596.x
27
Raggi P Chertow GM Torres PU Csiky B Naso A Nossuli K et al The ADVANCE Study: A Randomized Study to Evaluate the Effects of Cinacalcet Plus Low-Dose Vitamin D on Vascular Calcification in Patients on Hemodialysis. Nephrol Dial Transpl (2011) 26(4):1327–39. 10.1093/ndt/gfq725
28
Akizawa T Kurita N Mizobuchi M Fukagawa M Onishi Y Yamaguchi T et al PTH-Dependence of the Effectiveness of Cinacalcet in Hemodialysis Patients with Secondary Hyperparathyroidism. Sci Rep (2016) 6:19612. 10.1038/srep19612
29
Blair HA . Etelcalcetide: First Global Approval. Drugs (2016) 76(18):1787–92. 10.1007/s40265-016-0671-3
30
Akizawa T Ikejiri K Kondo Y Endo Y Fukagawa M . Evocalcet: A New Oral Calcimimetic for Dialysis Patients with Secondary Hyperparathyroidism. Ther Apher Dial (2020) 24(3):248–57. 10.1111/1744-9987.13434
31
Komaba H Nakanishi S Fujimori A Tanaka M Shin J Shibuya K et al Cinacalcet Effectively Reduces Parathyroid Hormone Secretion and Gland Volume Regardless of Pretreatment Gland Size in Patients with Secondary Hyperparathyroidism. Clin J Am Soc Nephrol (2010) 5(12):2305–14. 10.2215/CJN.02110310
32
Palmer SC Mavridis D Johnson DW Tonelli M Ruospo M Strippoli GFM . Comparative Effectiveness of Calcimimetic Agents for Secondary Hyperparathyroidism in Adults: A Systematic Review and Network Meta-Analysis. Am J Kidney Dis (2020) 76(3):321–30. 10.1053/j.ajkd.2020.02.439
33
Cianciolo G Tondolo F Barbuto S Angelini A Ferrara F Iacovella F et al A Roadmap to Parathyroidectomy for Kidney Transplant Candidates. Clin Kidney J (2022) 15(8):1459–74. 10.1093/ckj/sfac050
34
Yalla N Bobba G Guo G Stankiewicz A Ostlund R . Parathyroid Hormone Reference Ranges in Healthy Individuals Classified by Vitamin D Status. J Endocrinol Invest (2019) 42(11):1353–60. 10.1007/s40618-019-01075-w
35
Payne RB Little AJ Williams RB Milner JR . Interpretation of Serum Calcium in Patients with Abnormal Serum Proteins. Br Med J (1973) 4(5893):643–6. 10.1136/bmj.4.5893.643
36
Matsuo S Imai E Horio M Yasuda Y Tomita K Nitta K et al Revised Equations for Estimated GFR from Serum Creatinine in Japan. Am J Kidney Dis (2009) 53(6):982–92. 10.1053/j.ajkd.2008.12.034
37
Uemura O Nagai T Ishikura K Ito S Hataya H Gotoh Y et al Creatinine-Based Equation to Estimate the Glomerular Filtration Rate in Japanese Children and Adolescents with Chronic Kidney Disease. Clin Exp Nephrol (2014) 18(4):626–33. 10.1007/s10157-013-0856-y
38
Akizawa T Shimazaki R Fukagawa M , Evocalcet Study Group. Phase 2b Study of Evocalcet (KHK7580), a Novel Calcimimetic, in Japanese Patients with Secondary Hyperparathyroidism Undergoing Hemodialysis: A Randomized, Double-Blind, Placebo-Controlled, Dose-Finding Study. PLoS One (2018) 13(10):e0204896. 10.1371/journal.pone.0204896
39
Block GA Bushinsky DA Cheng S Cunningham J Dehmel B Drueke TB et al Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis with Secondary Hyperparathyroidism: A Randomized Clinical Trial. JAMA (2017) 317(2):156–64. 10.1001/jama.2016.19468
40
Pencina MJ Dagostino RB Dagostino RB Jr. Vasan RS . Evaluating the Added Predictive Ability of a New Marker: From Area under the ROC Curve to Reclassification and beyond. Stat Med (2008) 27(2):157–72. 10.1002/sim.2929
41
Pencina MJ D'Agostino RB Steyerberg EW . Extensions of Net Reclassification Improvement Calculations to Measure Usefulness of New Biomarkers. Stat Med (2011) 30(1):11–21. 10.1002/sim.4085
42
Alba AC Agoritsas T Walsh M Hanna S Iorio A Devereaux PJ et al Discrimination and Calibration of Clinical Prediction Models: Users' Guides to the Medical Literature. JAMA (2017) 318(14):1377–84. 10.1001/jama.2017.12126
43
Pencina MJ D'Agostino RB . Evaluating Discrimination of Risk Prediction Models: The C Statistic. JAMA (2015) 314(10):1063–4. 10.1001/jama.2015.11082
44
Babu GJ . Resampling Methods for Model Fitting and Model Selection. J Biopharm Stat (2011) 21(6):1177–86. 10.1080/10543406.2011.607749
45
Kanda Y . Investigation of the Freely Available Easy-To-Use Software 'EZR' for Medical Statistics. Bone Marrow Transpl (2013) 48(3):452–8. 10.1038/bmt.2012.244
46
Tominaga Y Kakuta T Yasunaga C Nakamura M Kadokura Y Tahara H . Evaluation of Parathyroidectomy for Secondary and Tertiary Hyperparathyroidism by the Parathyroid Surgeons' Society of Japan. Ther Apher Dial (2016) 20(1):6–11. 10.1111/1744-9987.12352
47
Goldfarb-Rumyantzev A Hurdle JF Scandling J Wang Z Baird B Barenbaum L et al Duration of End-Stage Renal Disease and Kidney Transplant Outcome. Nephrol Dial Transpl (2005) 20(1):167–75. 10.1093/ndt/gfh541
48
Lim JH Jeon Y Kim DG Kim YH Kim JK Yang J et al Effect of Pretransplant Dialysis Vintage on Clinical Outcomes in Deceased Donor Kidney Transplant. Sci Rep (2022) 12(1):17614. 10.1038/s41598-022-20003-2
49
Pirklbauer M Bushinsky DA Kotanko P Schappacher-Tilp G . Personalized Prediction of Short- and Long-Term PTH Changes in Maintenance Hemodialysis Patients. Front Med (Lausanne) (2021) 8:704970. 10.3389/fmed.2021.704970
50
Hiramitsu T Tomosugi T Okada M Futamura K Tsujita M Goto N et al Pre-Operative Localisation of the Parathyroid Glands in Secondary Hyperparathyroidism: A Retrospective Cohort Study. Sci Rep (2019) 9(1):14634. 10.1038/s41598-019-51265-y
Summary
Keywords
calcimimetics, kidney transplantation, parathyroidectomy, tertiary hyperparathyroidism, prediction model
Citation
Okada M, Sato T, Himeno T, Hasegawa Y, Futamura K, Hiramitsu T, Ichimori T, Goto N, Narumi S and Watarai Y (2024) Pre-Transplant Calcimimetic Use and Dose Information Improves the Accuracy of Prediction of Tertiary Hyperparathyroidism after Kidney Transplantation: A Retrospective Cohort Study. Transpl Int 37:12704. doi: 10.3389/ti.2024.12704
Received
18 January 2024
Accepted
18 April 2024
Published
01 May 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Okada, Sato, Himeno, Hasegawa, Futamura, Hiramitsu, Ichimori, Goto, Narumi and Watarai.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manabu Okada, ubanamadako@yahoo.co.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.