Dear Editors,
Kidney transplant recipients are high-risk cardiovascular patients and cardiovascular events are the most common cause of death after kidney transplantation [1]. Management of cardiovascular risk factors, which includes adequate lowering of LDL cholesterol (LDLC) to the recommended levels, is difficult to achieve after renal transplantation or is not implemented consistently often enough [2]. This is partly because immunosuppressive therapies such as tacrolimus, prednisolone, or everolimus themselves have adverse effects on lipid levels and partly because there are incompatibilities and interactions between statins and immunosuppressive drugs i.e., ciclosporin A that limit adequate statin therapy and ezetimibe administration [3, 4].
Therefore, novel and highly efficient therapies such as inclisiran (SmPC Leqvio, Novartis, Germany) may contribute to better LDLC management in this patient population. Inclisiran is a small interference-RNA against protein convertase subtilisin/kexin type 9 (PCSK9), preventing LDL receptor degradation [5]. It is injected subcutaneously at month 0 and 3 and every 6 months thereafter and results in ∼50% LDLC reduction [6]. Inclisiran was first approved in the European Union in December 2020 for the treatment of primary hypercholesterolemia or mixed dyslipidemia in combination with a statin or other lipid-lowering therapies in patients who do not achieve LDLC goals with the maximum tolerable statin dose, or alone or in combination with other lipid-lowering therapies in patients with statin intolerance or for whom a statin is contraindicated.
To our knowledge, there is no data about the use of inclisiran in kidney transplant recipients yet. Therefore, we present for the first time a case of a patient treated with inclisiran after renal transplantation.
Our 79-year-old male patient received a deceased donor kidney transplant 12 years prior to the first inclisiran administration. End-stage renal disease was caused by right-sided nephrectomy due to renal cell carcinoma and unspecified nephrosclerosis of the left kidney. The immunosuppressive regimen at the time reported consisted of everolimus and prednisolone, due to a history of CMV disease. Serum creatinine was 2.44 mg/dL with an estimated GFR of 24 mL/min/m2 (CKD4A2T, CKD EPI). The patient has a distinct cardiovascular risk profile. In addition to male sex and older age, he suffers from metabolic syndrome (mixed dyslipidemia, arterial hypertension, post-transplant diabetes mellitus, BMI of 25 kg/m2) with hyperuricemia and has a history of smoking (approximately 13 pack years). This has led to progressive peripheral artery disease (Fontaine IIB) and coronary artery disease.
Serum lipids were inadequately controlled during therapy with atorvastatin 80 mg and ezetimibe 10 mg daily (total cholesterol 5.18 mmol/L, LDLC 2.46 mmol/L, HDLC 2.12 mmol/L and triglycerides 1.79 mmol/L). For our very high-risk patient, the 2019 ESC/EAS guidelines on the treatment of dyslipidaemia recommend a target LDLC of < 1.4 mmol/L and an LDLC reduction >50% from baseline values [7]. Therapeutic options were discussed with the patient and the patient opted for inclisiran therapy for optimal therapy adherence.
Inclisiran (284 mg s.c.) was administered at 0 and 3 months and then every 6 months while continuing atorvastatin and ezetimibe. LDLC was significantly lowered to 1.03, 1.14, and 1.32 mmol/L after 6, 9 and 12 months, respectively (Figure 1).
FIGURE 1
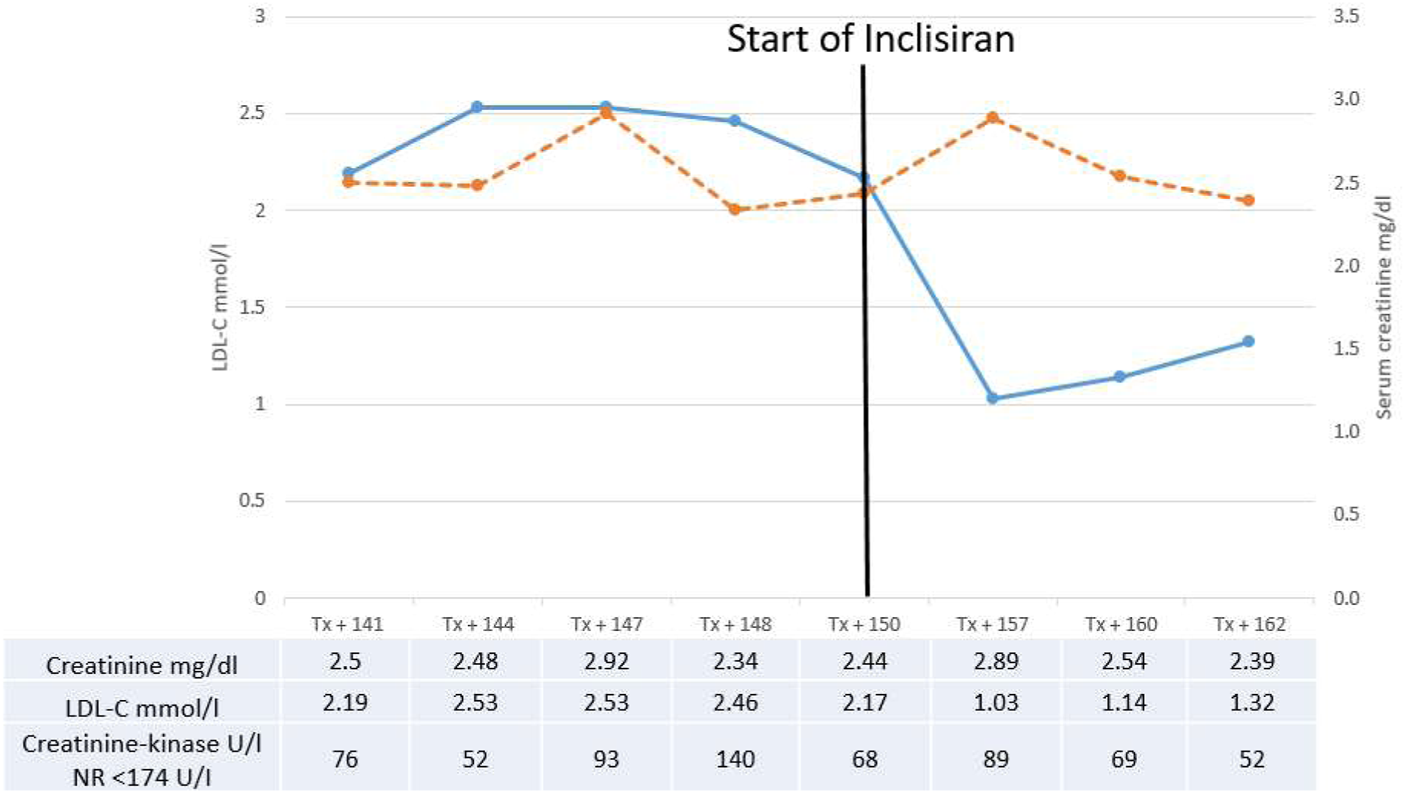
Serum-creatinine (dotted line) and LDL-cholesterol (line) before and after introduction of inclisiran therapy. Values of creatinine, LDL-cholesterol and creatinine-kinase are shown in the table. NR, normal range.
During the 1-year follow-up, renal function was stable after 12 months (serum creatinine 2.39 mg/dL, eGFR 25 mL/min/m2; Figure 1). We did not observe relevant side effects, or increase in proteinuria, creatinine-kinase or change in everolimus level.
The case presented demonstrates that inclisiran can be safely and conveniently administered with a profound effect on LDLC levels after renal transplantation. Further research needs to be conducted to demonstrate efficacy on cardiovascular death in transplanted patients.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: clinical data. Requests to access these datasets should be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LU and SR wrote the manuscript. LU, SR, and KG contributed data. UJ, HP, and KG revised the manuscript.
Funding
The APC has been funded by the Open Access Funds of the University of Münster.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Awan AA Niu J Pan JS Erickson KF Mandayam S Winkelmayer WC et al Trends in the Causes of Death Among Kidney Transplant Recipients in the United States (1996-2014). Am J Nephrol (2018) 48(6):472–81. 10.1159/000495081
2.
Thölking G Schulte C Jehn U Schütte-Nütgen K Pavenstädt H Suwelack B et al The Tacrolimus Metabolism Rate and Dyslipidemia after Kidney Transplantation. J Clin Med (2021) 10(14):3066. 10.3390/jcm10143066
3.
Ichimaru N Yamanaka K Kato T Kakuta Y Abe T Imamura R et al Risk Factors and Incidence for Lipid Abnormalities in Kidney Transplant Patients. Transpl Proc (2015) 47(3):672–4. 10.1016/j.transproceed.2014.12.029
4.
Simonson SG Raza A Martin PD Mitchell PD Jarcho JA Brown CD et al Rosuvastatin Pharmacokinetics in Heart Transplant Recipients Administered an Antirejection Regimen Including Cyclosporine. Clin Pharmacol Ther (2004) 76(2):167–77. 10.1016/j.clpt.2004.03.010
5.
Lambert G Charlton F Rye KA Piper DE . Molecular Basis of PCSK9 Function. Atherosclerosis (2009) 203(1):1–7. 10.1016/j.atherosclerosis.2008.06.010
6.
Wright RS Ray KK Raal FJ Kallend DG Jaros M Koenig W et al Pooled Patient-Level Analysis of Inclisiran Trials in Patients with Familial Hypercholesterolemia or Atherosclerosis. J Am Coll Cardiol (2021) 77(9):1182–93. 10.1016/j.jacc.2020.12.058
7.
Authors/Task Force MembersESC Committee for Practice Guidelines CPGESC National Cardiac Societies. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Atherosclerosis (2019) 290:140–205. 10.1016/j.atherosclerosis.2019.08.014
Summary
Keywords
kidney transplantation, renal transplantation, dyslipidemia, inclisiran, LDL
Citation
Ueberdiek L, Jehn U, Pavenstädt H, Gebauer K and Reuter S (2023) Novel Therapeutic Strategies for Dyslipidemia: First Report of Inclisiran Therapy in a Kidney Transplanted Patient. Transpl Int 36:11104. doi: 10.3389/ti.2023.11104
Received
05 December 2022
Accepted
18 January 2023
Published
26 January 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Ueberdiek, Jehn, Pavenstädt, Gebauer and Reuter.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Reuter, stefan.reuter@ukmuenster.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.