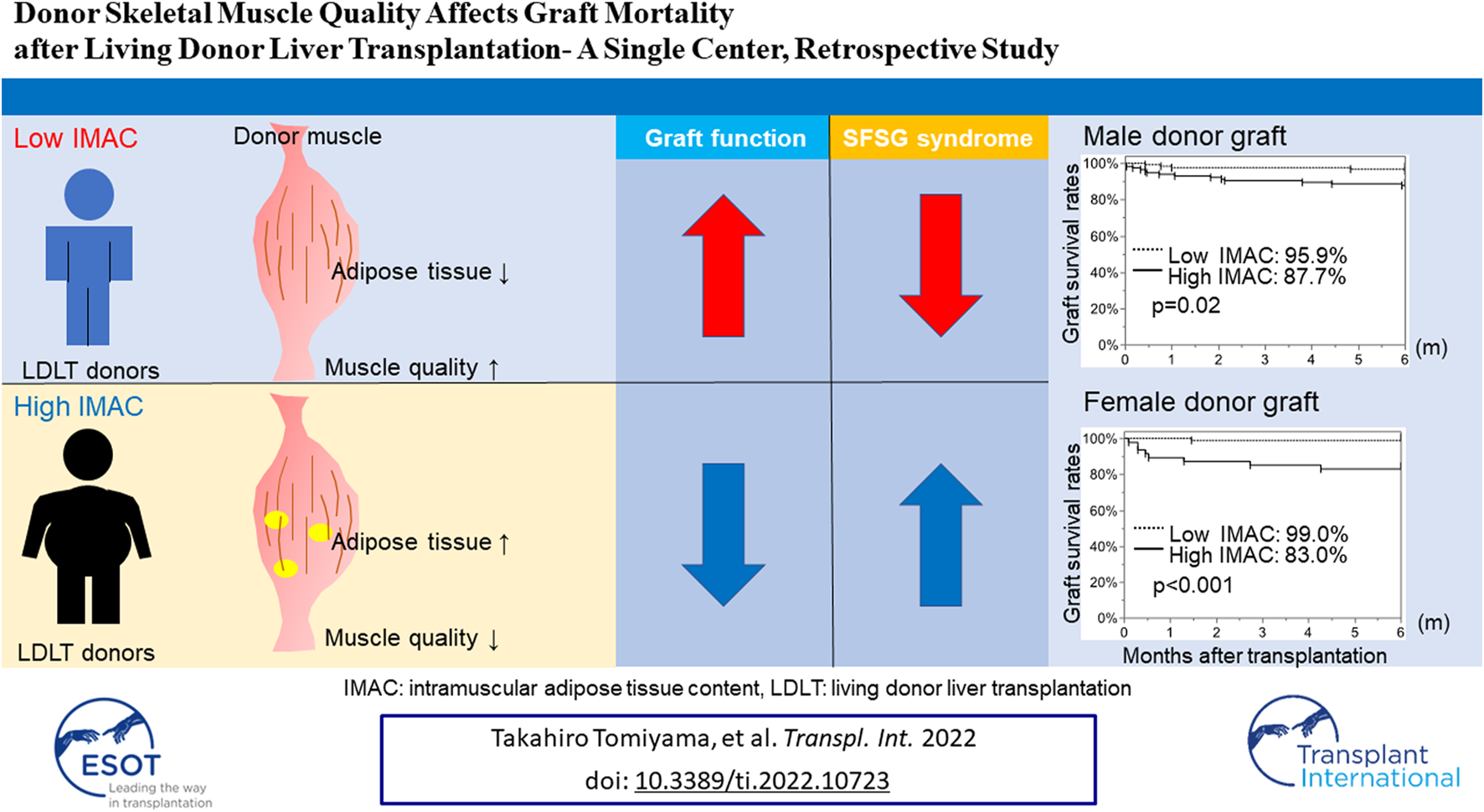
Abstract
The recipient muscle status is closely associated with postoperative poor survival in recipients of living donor liver transplantation (LDLT). However, it is uncertain whether LDLT donor muscle quality and quantity affect graft quality. Hence, we analyzed the correlation between donor muscle status and graft function. We measured the skeletal muscle mass index (SMI) and intramuscular adipose tissue content (IMAC) of 380 LDLT donors. We examined the correlation between donor SMI or IMAC and graft mortality, the occurrence rates of small-for-size graft (SFSG) syndrome, and 6-month graft survival rates. The donor SMI had no effect on the occurrence of SFSG syndrome and graft survival, while a high IMAC in both male and female donors was significantly correlated with the rate of SFSG syndrome [high vs low: (male donors) 15.8% vs. 2.5%, p = 0.0003; (female donors) 12.8% vs. 3.1%, p = 0.0234] and 6-month graft survival rates [(male donors) 87.7% vs 95.9%, p = 0.02; (female donors) 83.0% vs. 99.0%, p < 0.0001]. Multivariate analysis revealed that a high donor IMAC (HR; 5.42, CI; 2.13–13.8, p = 0.0004) was an independent risk factor for 6-month graft survival, and the donor IMAC is useful for donor selection for high-risk recipients.
Graphical Abstract
Introduction
Sarcopenia, defined as an age-dependent decrease in muscle mass and function, is reportedly an independent risk factor of poor survival in the presence of several diseases (1 –5). In the field of liver transplantation, preoperative recipient sarcopenia is reportedly correlated with increasing sepsis and mortality rates in recipients after living donor liver transplantation (LDLT) (6). In addition, the transplant recipient preoperative skeletal muscle mass-to-visceral fat area ratio, visceral adiposity, low muscularity, and high intramuscular adipose tissue content (IMAC) are closely associated with high postoperative recipient mortality following LDLT (7, 8). These findings indicate that low quantity and quality of muscle in the recipient preoperatively closely correlate with postoperative mortality in LDLT recipients.
However, these correlations with liver transplantation and muscle quality and quantity are not surprising because the liver is strongly affected by muscle tissue (9). Skeletal muscle tissue secretes a hormone, called myokine, which regulates muscle metabolism, increases insulin sensitivity, and influences adipose tissue mass and fat deposition in the liver (10,11). On the other hand, adipose tissue can release hormones, called adipokines, which regulate lipid metabolism, decrease insulin sensitivity, and influence fibrogenesis in the liver (10, 12). Thus, skeletal muscle is closely involved in determining the liver condition.
The effect of skeletal muscle in LDLT donors has not been fully examined. LDLT donors are healthy and lack severe comorbidities. Preoperative blood tests are performed to confirm that there are no abnormalities. In addition, donor liver steatosis and cold ischemic time are reportedly graft quality markers in deceased donor liver transplantation (DDLT) (13, 14). If a donor has mild obesity or fatty liver in LDLT, dietary restriction and exercise are implemented, and LDLT is performed after complete improvement of obesity and fatty liver. In LDLT, the cold ischemic time is very short and much less likely to be affected compared to DDLT. The population of LDLT donors is quite homogeneous compared to that of DDLT donors. However, there is diversity in LDLT donor body shape and muscle mass. In LDLT, exercise and diet improve the health of the donor (15), and regular exercise reduces intrahepatic adiposity, increases β-oxidation of fatty acids, and induces hepato-protective autophagy (16). Hence, donor muscularity may reflect the health status of the liver and the condition of the graft, and it may be useful to base the decision of donor selection in LDLT on donor muscularity.
In the present study, the pretransplant donor skeletal muscle mass index (SMI) and IMAC were retrospectively evaluated, and the impact of the SMI and IMAC on graft survival was assessed.
Methods
Patients
The study protocol was approved by the Institutional Review Board of the Kyushu University Hospital, approval number 2019–354. This study was conducted according to the Declaration of Helsinki of 1996. Written informed consent was obtained from all patients before LDLT. In total, 380 adult patients (age >17 years) underwent LDLT at Kyushu University Hospital, Japan, between January 2007 and March 2018. Recipients who could be followed for at least 6 months after LDLT were included. If an LDLT donor had mild obesity or fatty liver, dietary restriction and exercise were implemented, and LDLT was performed after complete improvement of obesity and fatty liver. In our cohort, only two donors underwent weight loss before the donation.
Image Analysis
Computed tomography (CT) scanning was performed within 1 month preoperatively. The SMI and IMAC were calculated as previously reported (5, 17). Briefly, the skeletal muscle area and IMAC were calculated using cross-sectional CT images obtained at the third lumbar vertebral level. Skeletal muscle areas were measured by manual tracing and normalized with patient height in m2 and expressed as the SMI (Figure 1A). The preoperative mean CT value for the right and left multifidus muscles (in Hounsfield units, HU) was divided by the mean CT value for four points of subcutaneous fat and expressed as the IMAC (Figure 1B). A higher IMAC indicates a larger amount of adipose tissue in the skeletal muscle and, therefore, muscle that is of poorer quality.
FIGURE 1
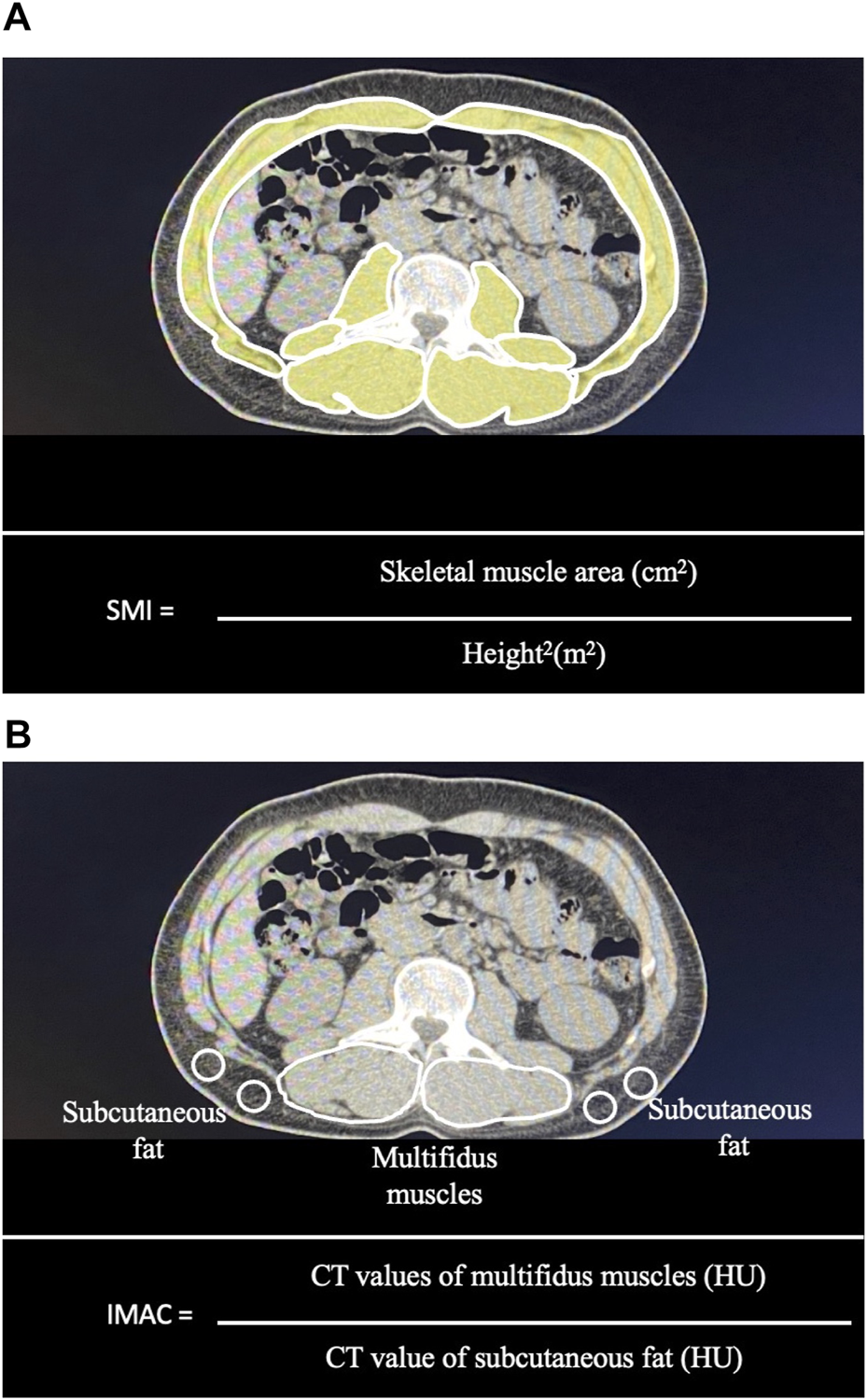
Measurement of the skeletal muscle mass index (SMI) and intramuscular adipose tissue content (IMAC) using a cross-sectional CT image obtained at the third lumbar vertebral level: (A) Skeletal muscle areas were measured by manual tracing. Skeletal muscle areas were normalized with patient height in m2 and expressed as the SMI. (B) The preoperative mean CT value for the right and left multifidus muscles (in Hounsfield units, HU) was divided by the mean CT value for four points of subcutaneous fat and expressed as the IMAC.
Selection Criteria
The selection criteria for the recipients and donors have been previously described (18, 19).
The selection criteria for LDLT for patients without hepatocellular carcinoma (HCC) were as follows: 1) no other potentially curative modality available and 2) no other organ failure present. There was no limitation on recipient age. The selection criteria for LDLT for patients with HCC were as follows: 1) no other potentially curative modality available, 2) no extrahepatic metastasis, and 3) no major vascular invasion. The Model for End-Stage Liver Disease (MELD) score was calculated using a formula reported by Kamath et al. (20).
Donors were selected from among candidates who had volunteered for the procedure (18). They were required to be within three degrees of consanguinity or the spouse of the recipient and to be between 20 and 65 years of age. For donors not within three degrees of consanguinity with the recipient, individual approval was obtained from the Ethic Committee of Kyushu University Hospital. Good Samaritan donation was not used. The standard liver volume of recipients was calculated according to the formula of Urata (21). Three-dimensional CT was performed for volumetric analysis and delineation of vascular anatomy. Decisions regarding graft type were based on the preoperatively predicted graft volume/recipient standard liver volume (GV/SLV) ratio. Left lobe + caudate lobe grafts were basically used when the preoperatively predicted GV/SLV ratio was ≥35%, but relatively small grafts, such as those with a GV/SLV between 30% and 35%, were selected when the donor was younger than 30 years of age (18). When the GV/SLV ratio of the left lobe + caudate lobe graft was <35% and remnant liver volume after right lobectomy was ≥35%, a right lobe graft was used. A posterior segment graft was considered when the donor’s vascular anatomy was suitable for this purpose (22).
Surgical Technique
The graft procurement technique and recipient surgery have been previously described (23). Splenectomy was performed using a vessel sealing system (Ligasure; Covidien Japan, Tokyo, Japan) and automatic suturing device (Endo GIA, Covidien Japan or Powered ECHELON, ETHICON, New Brunswick, NJ, United States) as described previously (19, 24).
Postoperative Management
The perioperative management of recipients, including the immunosuppression regimens, have been described previously (18, 19, 25). Briefly, immunosuppression was initiated using a protocol based on either tacrolimus (Prograf: Astellas Pharma, Tokyo, Japan) or cyclosporine A (Neoral; Novartis Pharma K.K, Tokyo, Japan) with mycophenolate mofetil (CellCept; Pfizer, New York, America) and steroids. The target trough concentration for tacrolimus was set at 10 ng/ml for 3 months after LDLT, followed by 5–10 ng/ml. The target trough concentration for cyclosporine A was set at 250 ng/ml for 3 months after LDLT, followed by 150–200 ng/ml. Methylprednisolone was initiated on the day of the LDLT, after which the dose was tapered, and prednisolone was sustained 7 days after the LDLT. Prednisolone treatment was tapered and discontinued 6 months after LDTL. Mycophenolate mofetil was used, beginning with 2 or 3 g on the day after LDLT; the dose was tapered and discontinued 6 months after LDLT. The trough concentration of mycophenolate mofetil was not measured.
Portal, hepatic arterial, and hepatic venous flows were assessed using Doppler ultrasonography twice per day until postoperative-day (POD) 7 and once per day thereafter during the first admission. For recipients with simultaneous splenectomy and LDLT, portal vein thrombosis prevention was not routinely performed. When the platelet count increased to 500,000/ml or higher during the follow-up period, 100 mg of aspirin was administered, which was discontinued when the platelet count decreased below 500,000/ml.
The abdominal drain was removed when the daily ascites volume became lower than 500 ml.
Endpoints
The primary endpoint was 6-month graft survival. If there was a significant difference in the first endpoint, we also examined laboratory data and the amount of abdominal drainage as secondary endpoints. Six-month graft loss was defined as recipient death or re-transplantation within 6 months.
Parameters Analyzed
Data Analysis
Categorical variables are presented as numbers and percentages and all patient background information was compared using the Pearson’s chi-square test. Based on their distributions, continuous variables are presented as the mean with 95% confidence interval, and they were compared using the t-test.
Graft survival data were analyzed using the Kaplan–Meier method and compared using the log-rank test. Continuous variables were compared using the t-test and categorical variables were compared using the chi-squared (?2) test. Any variable in the univariate analysis was identified as significant at p < 0.05, or variables at p < 0.2 were considered candidates for the multivariate Cox analysis. The results are shown as hazard ratios (HRs) with 95% confidence intervals (CIs). A value of p < 0.05 was considered to indicate statistical significance. All statistical data were generated using JMP Pro 15 (SAS Institute, Cary, NC, United States).
Results
Measurement of Donor SMI and IMAC and Examination of the Cutoff Value
There was a significant difference in donor SMI and IMAC by sex [male donor (n = 235) vs. female donor (n = 145); mean SMI: 50.1 vs. 39.4, p < 0.0001, mean IMAC: −0.557 vs. −0.507, p < 0.0001]. Thus, we separated data from male and female donors for further analysis. The optimal cutoff values for predicting primary 6-month graft loss were derived from receiver operating characteristic curves, with SMI cutoff values for men and women of 57.0 and 37.5 (sensitivity 31.6% and 77.8%, respectively; specificity 87.5% and 59.6%, respectively), and IMAC cut-off values for men and women of −0.553 and −0.473, respectively (sensitivity 73.7% and 88.9%, respectively; specificity 53.2% and 71.3%, respectively) (Figures 2A–D).
FIGURE 2
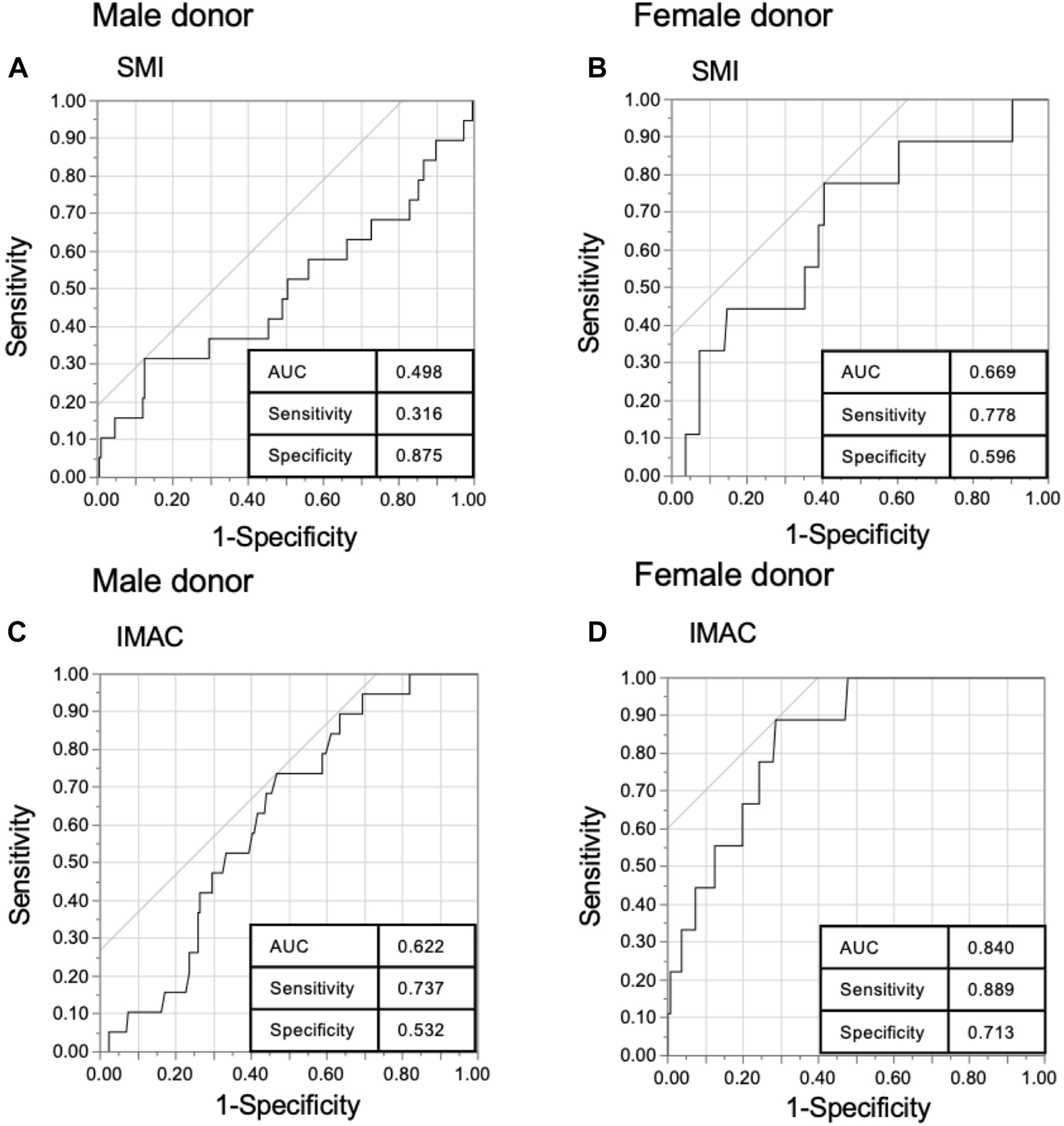
Receiver operating characteristics (ROC) curve of the donor muscle mass index (SMI) and intramuscular adipose tissue content (IMAC). ROC of (A) male donor SMI, (B) female donor SMI, (C) male donor IMAC, and (D) female donor IMAC with 6-month graft survival in living donor liver transplantation.
Correlation of Preoperative Donor Muscle Condition With Patient Characteristics
As shown in Table 1, depicting the male donor SMI analysis, there were significant differences in the rates of right lobe grafts and sepsis after transplantation between the low-SMI and high-SMI groups (low-SMI group vs. high-SMI group; right lobe rate: 58.3% vs. 76.7%, p = 0.0248, sepsis rate: 6.3% vs. 16.3%, p = 0.0292). Table 2 shows the patient characteristics in female donors. Cold ischemic time was significantly longer in the low-SMI group than in the high-SMI group (157 min vs. 113 min, p = 0.0013), but there was no significant difference in recipient postoperative parameters. Table 3 shows the patient characteristics for the male donor IMAC analysis. In the high-IMAC group, the rates of right lobe graft and GV/SLV were lower (38.2% vs. 40.8%, p = 0.0283) and intraoperative recipient blood loss was higher (3.90L vs. 3.79L, p = 0.0362) than in the low-IMAC group. Regarding recipient postoperative parameters, the admission period was longer (29 vs. 24 days, p = 0.0492) and the rates of SFSG syndrome (15.8% vs. 2.5%, p = 0.0004) and graft failure (12.3% vs. 4.13%, p = 0.0220) were higher in the high-IMAC than in the low-IMAC group. Table 4 shows the patient characteristics for the female donor IMAC analysis. The rate of ABO incompatibility was lower (8.51% vs. 22.5%, p = 0.0406) and the MELD score was higher (19 vs. 16, p = 0.0005) in the high-IMAC group than in the low-IMAC group. Regarding postoperative parameters, the rates of sepsis, SFSG syndrome, and graft failure were higher in the high donor IMAC group than in the low donor IMAC group (sepsis; 14.9% vs. 2.41%, p = 0.0027; SFSG syndrome; 12.8% vs. 3.1%, p = 0.0234; graft failure within 6-month; 17.0% vs. 1.02%). In both the male and female analyses, there was no significant difference in the cause of graft loss.
TABLE 1
| Variables | Male donor SMI | p-value | |
|---|---|---|---|
| High (n = 43) | Low (n = 192) | ||
| Preoperative donor variables | |||
| Age (years) | 33 (20–62) | 36 (20–63) | 0.0636 |
| Graft (right lobe) | 10 (23.3%) | 80 (41.7%) | 0.0248 |
| Actual GV/SLV (%) | 40.3 (23.2–73.1) | 39.8 (22.6–70.1) | 0.7135 |
| Actual GRWR (%) | 0.787 (0.430–1.35) | 0.770 (0.397–1.42) | 0.7616 |
| ABO (Incompatible) | 5 (11.6%) | 32 (16.7%) | 0.4122 |
| Recipient preoperative variables | |||
| Age (years) | 55 (19–76) | 57 (20–74) | 0.3485 |
| Sex (male) | 15 (34.9%) | 78 (40.6%) | 0.4865 |
| Primary diagnosis | |||
| Hepatocellular disease | 30 (69.8%) | 130 (67.7%) | |
| Cholestatic disease | 20 (20.9%) | 32 (16.7%) | |
| Others | 4 (9.30%) | 30 (15.6%) | |
| HBsAb (yes) | 9 (20.9%) | 50 (26.0%) | 0.507 |
| HCVAb (yes) | 18 (41.9%) | 70 (36.5%) | 0.5082 |
| HCC (yes) | 19 (44.2%) | 76 (39.6%) | 0.5783 |
| Body mass index (kg/m2) | 24.1 (15.8–32.0) | 23.7 (14.9–35.6) | 0.9234 |
| ICU or hospital statement (yes) | 14 (32.6%) | 67 (34.9%) | 0.7706 |
| DM (yes) | 10 (23.3%) | 38 (19.8%) | 0.6105 |
| MELD | 14 (4–29) | 15 (4–54) | 0.076 |
| Splenectomy (yes) | 32 (74.4%) | 163 (84.9%) | 0.0985 |
| Pre-transplant WBC count (x103/μL) | 4.06 (1.46–15.7) | 4.06 (0.39–20.6) | 0.5546 |
| Pre-transplant Platelet count (x104/μL) | 7.40 (2.60–44.6) | 6.85 (0.90–30.2) | 0.071 |
| Intraoperative parameters | |||
| Recipient operation time (h) | 12.2 (8.33–21.6) | 12.1 (7.55–24.8) | 0.7576 |
| Recipient blood loss (L) | 3.80 (0.15–68.3) | 3.84 (0.12–220) | 0.7711 |
| Cold ischemic time (min) | 81.5 (42–210) | 92 (35–376) | 0.055 |
| Warm ischemic time (min) | 38 (28–125) | 41 (25–119) | 0.6401 |
| PVP before the end of operation | 16 (9–25) | 16 (6–30%) | 0.6908 |
| Recipient postoperative parameter | |||
| Admission period (day) | 25 (13–78) | 25 (1–145) | 0.497 |
| Sepsis | 7 (16.3%) | 12 (6.3%) | 0.0292 |
| SFSG syndrome | 3 (7.0%) | 18 (9.4%) | 0.6183 |
| Acute cellular or humoral rejection | 7 (16.3%) | 18 (9.38%) | 0.1844 |
| Graft failure within 6 months | 5 (11.6%) | 14 (7.29%) | 0.3458 |
| Cause of graft loss within 6 months | |||
| Liver failure | 2 (40.0%) | 8 (57.1%) | |
| Sepsis | 1 (20.0%) | 4 (28.6%) | |
| Others | 2 (20.0%) | 2 (14.3%) | 0.4805 |
Difference in patient characteristic between male high SMI group and low SMI group.
Data are presented as median (range) or n (%).
DM, diabetes mellitus; GRWR, graft recipient weight ratio; GV/SLV, graft volume/recipient standard liver volume ratio; HBsAg, hepatitis B surface antigen, HCC, hepatocellular carcinoma; HCVAb, hepatitis C virus antibody; HCC, hepatocellular carcinoma; ICU, intensive care unit; MELD, Model for End-Stage Liver Disease; PVP, portal vein pressure; SFSG, small-for-size graft.
TABLE 2
| Variables | Female donor SMI | p-value | |
|---|---|---|---|
| High (n = 89) | Low (n = 56) | ||
| Preoperative donor variables | |||
| Age (years) | 38 (21–64) | 39 (21–62) | 0.2019 |
| Graft (right lobe) | 61 (68.5%) | 45 (80.4%) | 0.1182 |
| Actual GV/SLV (%) | 42.5 (26.9–63.0) | 40.6 (28.9–56.4) | 0.2386 |
| Actual GRWR (%) | 0.782 (0.524–1.21) | 0.755 (0.509–1.19) | 0.3869 |
| ABO (Incompatible) | 17 (19.1%) | 9 (16.7%) | 0.6433 |
| Recipient preoperative variables | |||
| Age (years) | 56 (17–73) | 71 (21–71) | 0.4245 |
| Sex (male) | 39 (43.8%) | 33 (58.9%) | 0.0765 |
| Primary diagnosis | |||
| Hepatocellular disease | 62 (70.0%) | 36 (64.3%) | 0.3052 |
| Cholestatic disease | 20 (22.5%) | 11 (19.6%) | |
| Others | 7 (7.87%) | 9 (16.1%) | |
| HBsAb (yes) | 14 (15.7%) | 10 (17.9%) | 0.7372 |
| HCVAb (yes) | 28 (31.5%) | 15 (26.8%) | 0.5484 |
| HCC (yes) | 24 (27.0%) | 17 (30.4%) | 0.6589 |
| Body mass index (kg/m2) | 23.3 (17.0–32.9) | 23.3 (17.2–29.0) | 0.433 |
| ICU or hospital statement (yes) | 25 (28.4%) | 21 (37.5%) | 0.254 |
| DM (yes) | 10 (11.2%) | 8 (14.3%) | 0.5876 |
| MELD | 16 (5–44) | 17 (5–45) | 0.2161 |
| Splenectomy (yes) | 78 (87.6%) | 48 (85.7%) | 0.7379 |
| Pre-transplant WBC count (x103/μL) | 3.99 (1.04–15.9) | 4.20 (1.17–15.8) | 0.9446 |
| Pre-transplant Platelet count (x104/μL) | 7.00 (1.2–36.2) | 6.25 (1.7–34.8) | 0.7092 |
| Intraoperative parameters | |||
| Recipient operation time (h) | 12.6 (8.10–18.0) | 12.5 (8.47–20.7) | 0.2898 |
| Recipient blood loss (L) | 3.70 (0.58–26.4) | 5.62 (0.20–50.4) | 0.077 |
| Cold ischemic time (min) | 113 (39–261) | 157 (50–367) | 0.0013 |
| Warm ischemic time (min) | 41 (25–103) | 44.5 (22–83) | 0.4413 |
| PVP before the end of operation | 15 (7–25) | 14.5 (9–22) | 0.5789 |
| Recipient postoperative parameter | |||
| Admission period (day) | 27 (9–172) | 29 (3–80) | 0.289 |
| Sepsis | 5 (5.62%) | 4 (7.14%) | 0.711 |
| SFSG syndrome | 6 (6.7%) | 3 (5.4%) | 0.7366 |
| Acute cellular or humoral rejection | 7 (7.87%) | 5 (8.93%) | 0.821 |
| Graft failure within 6 months | 7 (7.87%) | 2 (3.57%) | 0.2968 |
| Cause of graft loss within 6 months | |||
| Liver failure | 3 (42.9%) | 2 (100%) | 0.1515 |
| Sepsis | 4 (57.1%) | 0 (0%) | |
| Others | 0 (0%) | 0 (0%) | |
Difference in patient characteristic between female high SMI group and low SMI group.
Data are presented as median (range) or n (%).
DM, diabetes mellitus; GRWR, graft recipient weight ratio; GV/SLV, graft volume/recipient standard liver volume ratio; HBsAg, hepatitis B surface antigen, HCC, hepatocellular carcinoma; HCVAb, hepatitis C virus antibody; HCC, hepatocellular carcinoma; ICU, intensive care unit; MELD, Model for End-Stage Liver Disease; PVP, portal vein pressure; SFSG, small-for-size graft.
TABLE 3
| Variables | Male donor IMAC | p-value | |
|---|---|---|---|
| Low (n = 121) | High (n = 114) | ||
| Preoperative donor variables | |||
| Age (years) | 34 (20–63) | 37 (20–62) | 0.1697 |
| Graft (right lobe) | 54 (44.6%) | 36 (31.6%) | 0.0397 |
| Actual GV/SLV (%) | 40.8 (26.8–70.1) | 38.2 (22.6–73.1) | 0.0283 |
| ABO (Incompatible) | 0.792 (0.482–1.42) | 0.737 (0.397–1.35) | 0.0552 |
| Recipient preoperative variables | 17 (14.1%) | 20 (17.5%) | 0.4623 |
| Age (years) | 55 (22–74) | 57 (19–76) | 0.6764 |
| Sex (male) | 54 (44.6%) | 39 (34.2%) | 0.1026 |
| Primary diagnosis | |||
| Hepatocellular disease | 86 (71.1%) | 74 (64.9%) | 0.5522 |
| Cholestatic disease | 20 (16.5%) | 21 (18.4%) | |
| Others | 15 (12.4%) | 19 (16.7%) | |
| HBsAb (yes) | 28 (23.1%) | 31 (27.2%) | 0.474 |
| HCVAb (yes) | 47 (38.8%) | 41 (36.0%) | 0.6487 |
| HCC (yes) | 53 (43.8%) | 42 (36.8%) | 0.2773 |
| Body mass index (kg/m2) | 23.7 (14.9–35.0) | 24.1 (15.8–35.6) | 0.7142 |
| ICU or hospital statement (yes) | 41 (33.9%) | 40 (35.1%) | 0.8462 |
| DM (yes) | 40 (15.8%) | 19 (19.6%) | 0.3982 |
| MELD | 15 (4–54) | 15 (4–39) | 0.8274 |
| Splenectomy (yes) | 102 (84.3%) | 93 (81.6%) | 0.5794 |
| Pre-transplant WBC count (x103/μL) | 4.10 (0.39–16.1) | 3.96 (0.96–20.6) | 0.5805 |
| Pre-transplant Platelet count (x104/μL) | 6.9 (1.8–41.5) | 7.0 (0.9–44.6) | 0.5164 |
| Intraoperative parameters | |||
| Recipient operation time (h) | 12.2 (7.55–23.1) | 12.0 (7.57–24.9) | 0.3754 |
| Recipient blood loss (L) | 3.79 (0.14–29.9) | 3.90 (0.12–22.0) | 0.0362 |
| Cold ischemic time (min) | 88 (35–313) | 89 (38–376) | 0.9574 |
| Warm ischemic time (min) | 41 (26–104) | 40.5 (25–125) | 0.5239 |
| PVP before the end of operation | 15 (6–30) | 16 (8–26) | 0.1224 |
| Recipient postoperative parameter | |||
| Admission period (day) | 24 (9–145) | 29 (1–133) | 0.0492 |
| Sepsis | 7 (5.79%) | 12 (10.5%) | 0.1827 |
| SFSG syndrome | 3 (2.5%) | 18 (15.8%) | 0.0004 |
| Acute cellular or humoral rejection | 13 (10.7%) | 12 (10.5%) | 0.9569 |
| Graft failure within 6 months | 5 (4.13%) | 14 (12.3%) | 0.022 |
| Cause of graft loss within 6 months | |||
| Liver failure | 3 (60.0%) | 7 (50.0%) | |
| Sepsis | 1 (20.0%) | 4 (28.6%) | |
| Others | 1 (20.0%) | 3 (21.4%) | 0.9156 |
Difference in patient characteristic between male high IMAC group and low IMAC group.
Data are presented as median (range) or n (%).
DM, diabetes mellitus; GRWR, graft recipient weight ratio; GV/SLV, graft volume/recipient standard liver volume ratio; HBsAg, hepatitis B surface antigen, HCC, hepatocellular carcinoma; HCVAb, hepatitis C virus antibody; HCC, hepatocellular carcinoma; ICU, intensive care unit; MELD, Model for End-Stage Liver Disease; PVP, portal vein pressure; SFSG, small-for-size graft.
TABLE 4
| Variables | Female donor IMAC | p-value | |
|---|---|---|---|
| Low (n = 98) | High (n = 47) | ||
| Preoperative donor variables | |||
| Age (years) | 37 (21–60) | 40 (20–64) | 0.2281 |
| Graft (right lobe) | 72 (73.5%) | 34 (72.3%) | 0.8859 |
| Actual GV/SLV (%) | 40.7 (26.9–63.0) | 42.5 (28.0–54.8) | 0.5307 |
| Actual GRWR (%) | 0.760 (0.509–1.22) | 0.783 (0.563–1.16) | 0.6636 |
| ABO (Incompatible) | 22 (22.5%) | 4 (8.51%) | 0.0406 |
| Recipient preoperative variables | |||
| Age (years) | 57 (23–71) | 58 (17–73) | 0.995 |
| Sex (male) | 48 (49.0%) | 24 (51.1%) | 0.8143 |
| Primary diagnosis | |||
| Hepatocellular disease | 63 (64.3%) | 35 (74.5%) | |
| Cholestatic disease | 27 (27.6%) | 4 (8.51%) | |
| Others | 8 (8.16%) | 8 (17.0%) | 0.0171 |
| HBsAb (yes) | 16 (16.3%) | 8 (17.2%) | 0.9161 |
| HCVAb (yes) | 28 (28.6%) | 15 (31.9%) | 0.6799 |
| HCC (yes) | 25 (25.5%) | 16 (34.0%) | 0.2856 |
| Body mass index (kg/m2) | 23.5 (17.0–30.4) | 23.0 (17.2–32.9) | 0.8357 |
| ICU or hospital statement (yes) | 30 (30.9%) | 16 (30.0%) | 0.707 |
| DM (yes) | 12 (12.2%) | 6 (12.7%) | 0.929 |
| MELD | 16 (5–36) | 19 (9–45) | 0.0005 |
| Splenectomy (yes) | 84 (85.7%) | 42 (89.4%) | 0.5424 |
| Pre-transplant WBC count (x103/μL) | 4.03 (1.06–15.8) | 4.05 (1.04–15.9) | 0.0799 |
| Pre-transplant Platelet count (x104/μL) | 7.05 (1.2–36.2) | 5.70 (1.2–24.3) | 0.208 |
| Intraoperative parameters | |||
| Recipient operation time (h) | 12.5 (8.10–20.7) | 12.6 (9.33–19.3) | 0.2366 |
| Recipient blood loss (L) | 3.93 (0.45–50.4) | 4.42 (0.20–34.7) | 0.7987 |
| Cold ischemic time (min) | 129 (39–367) | 151 (50–255) | 0.5455 |
| Warm ischemic time (min) | 43 (23–103) | 40 (22–83) | 0.2999 |
| PVP before the end of operation | 15 (7–25) | 15 (7–24) | 0.6381 |
| Recipient postoperative parameter | |||
| Admission period (day) | 27 (9–172) | 30 (3–128) | 0.2483 |
| Sepsis | 2 (2.04%) | 7 (14.9%) | 0.0027 |
| SFSG syndrome | 3 (3.1%) | 6 (12.8%) | 0.0234 |
| Acute cellular or humoral rejection | 10 (10.2%) | 2 (4.26%) | 0.2236 |
| Graft failure within 6 months | 1 (1.02%) | 8 (17.0%) | 0.0002 |
| Cause of graft loss within 6 months | |||
| Liver failure | 1 (100%) | 4 (50%) | |
| Sepsis | 0 (0%) | 4 (50%) | |
| Others | 0 (0%) | 0 (0%) | 0.3428 |
Difference in patient characteristic between female high IMAC group and low IMAC group.
Data are presented as median (range) or n (%).
DM, diabetes mellitus; GRWR, graft recipient weight ratio; GV/SLV, graft volume/recipient standard liver volume ratio; HBsAg, hepatitis B surface antigen, HCC, hepatocellular carcinoma; HCVAb, hepatitis C virus antibody; HCC, hepatocellular carcinoma; ICU, intensive care unit; MELD, Model for End-Stage Liver Disease; PVP, portal vein pressure; SFSG, small-for-size graft.
In male donors, there was a significant negative correlation between preoperative donor SMI and donor age (Figure 3A, r = −0.1289, p = 0.0484), while for female donors, there was no correlation between the SMI and age (Figure 3B, r = −0.0392, p = 0.6400). In all donors, there were significant positive correlations between the preoperative donor IMAC and donor age (Figures 3C,D; male; r = 0.1340, p = 0.0401; female; r = 0.1792, p = 0.0310).
FIGURE 3
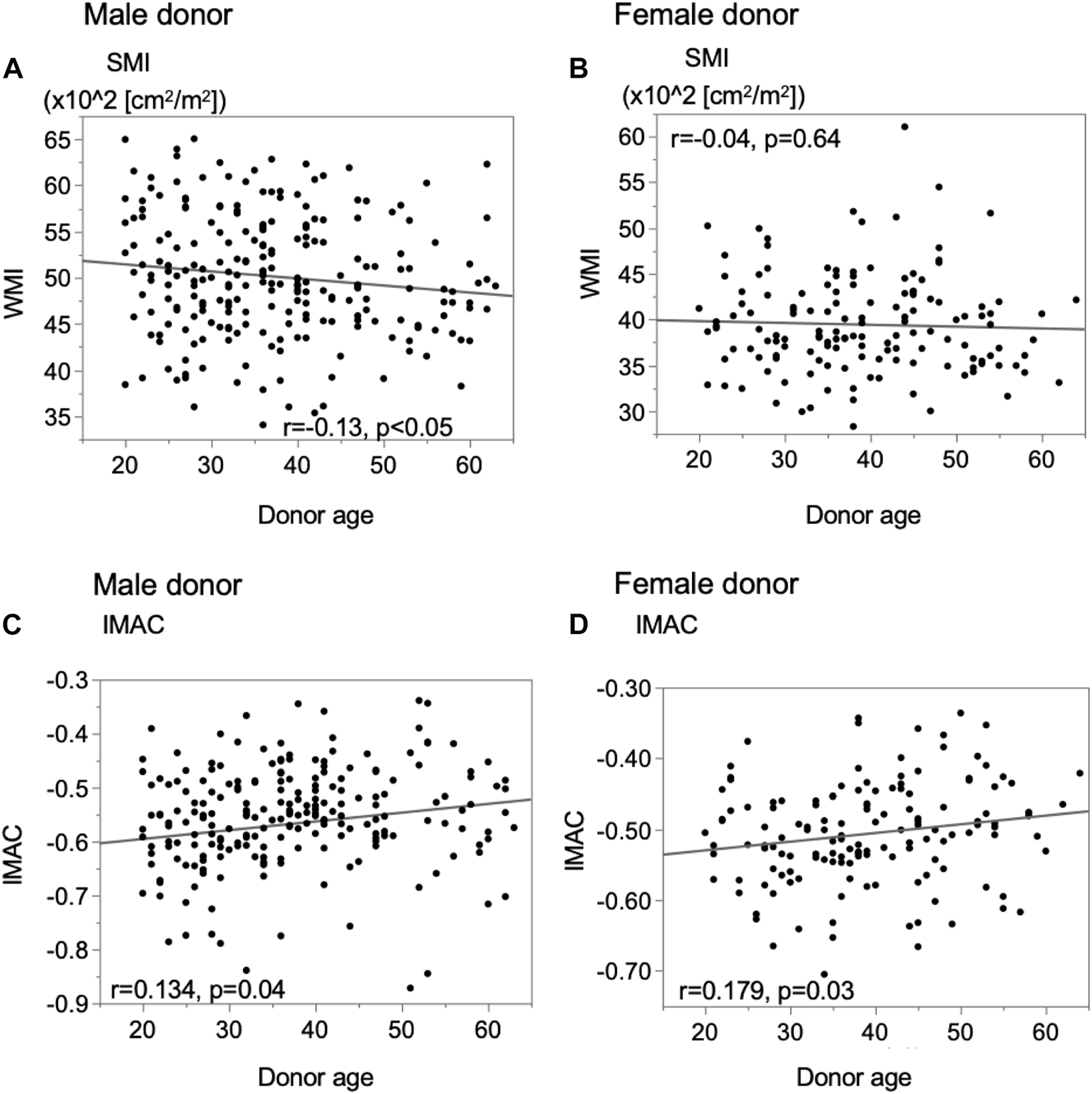
Analysis of the correlation between donor muscle status and donor age. (A) Male donor SMI, (B) female donor SMI, (C) male donor IMAC, and (D) female donor IMAC. SMI; muscle mass index, IMAC; intramuscular adipose tissue content.
Comparison of Graft Function
The male and female SMI analyses revealed no significant difference in the overall graft survival rates within 6 months after LDLT between the low-SMI group and the high-SMI group (Figures 4A,B; male; 92.3% vs. 88.4%, p = 0.3570; female; 96.4% vs. 91.1%, p = 0.3128). The male and female IMAC analyses showed that the overall graft survival rates in the high-IMAC group were lower than those in the low-IMAC group (Figures 4C,D; male; 87.7% vs. 95.9%, p = 0.0210; female; 83.0% vs. 99.0%, p < 0.0001).
FIGURE 4
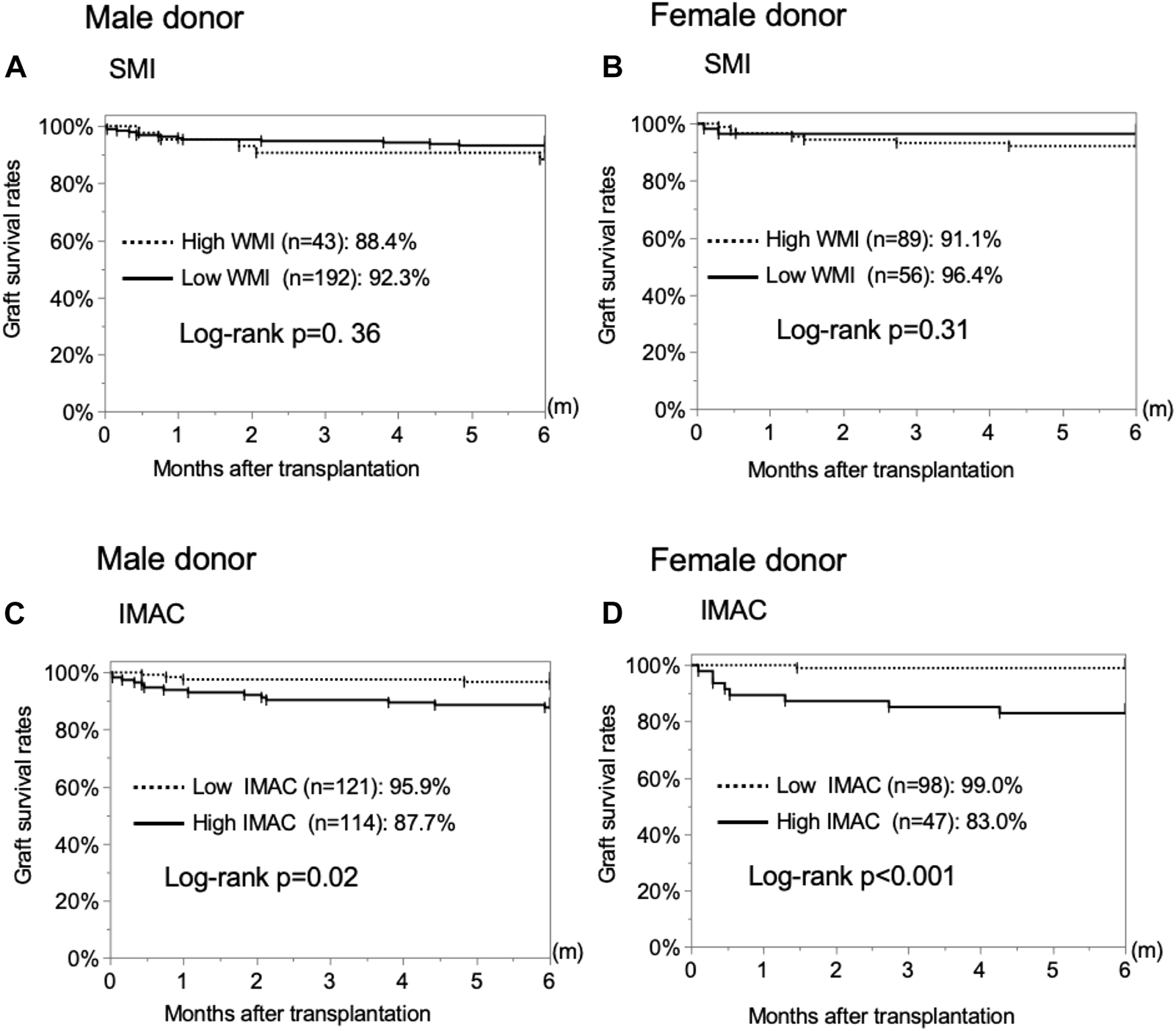
Analysis of the correlation between donor muscle status and 6-month graft survival rates. (A) Male donor SMI, (B) female donor SMI, (C) male donor IMAC, and (D) female donor IMAC. SMI, muscle mass index; IMAC, intramuscular adipose tissue content.
The differences in graft function after LDLT between the high- and low-IMAC groups were examined. The serum total-bilirubin (T-bil) level on POD 14, prothrombin-time international normalized ratio (PT-INR) on POD 14, and drained ascites on PODs 14 and 30 were significantly higher in recipients with grafts from high-IMAC donors than from low-IMAC donors (Figures 5A–D; T-bil; 6.2 mg/dl vs. 4.5 mg/dl, p = 0.0042; PT-INR: 1.15 vs. 1.10, p = 0.0043; ascites on POD 14: 425 ml vs. 228 ml, p = 0.0030; ascites on POD 30: 95 ml vs. 41 ml, p = 0.0355). We examined liver steatosis in 186 LDLT donors with preserved liver biopsy tissue using hematoxylin and eosin staining to assess the correlation between liver steatosis and IMAC. There were 88 high-IMAC patients and 98 low-IMAC patients. No patient had 5% or higher steatosis in either group. There was no significant difference between the groups in the rate of donors with microvascular steatosis (1%–4%) (high IMAC vs. low IMAC: 13.6% vs. 11.2%, p = 0.6179).
FIGURE 5
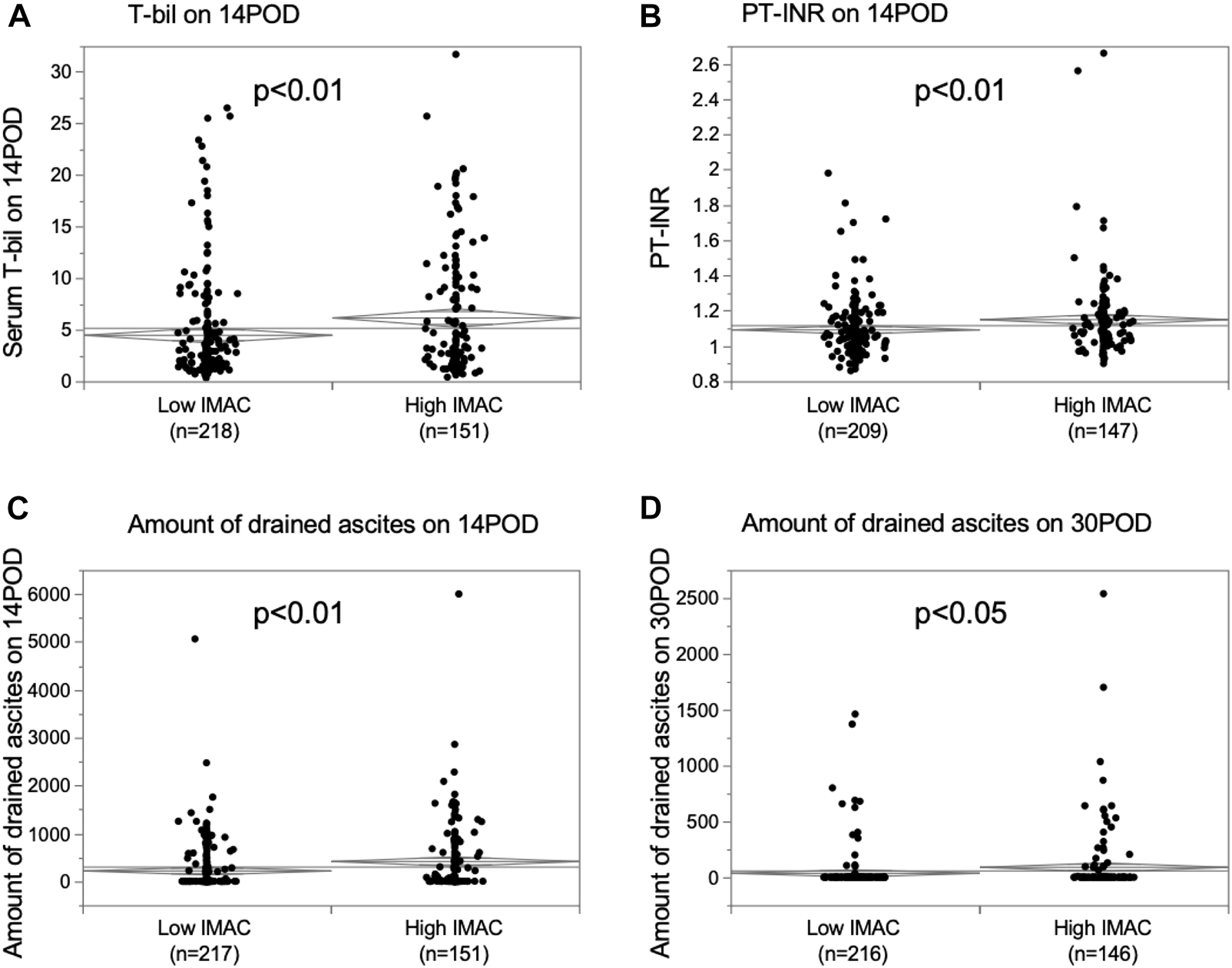
Comparison of graft function in recipients with high IMAC graft and low IMAC graft. (A) Serum T-bil on POD 14, (B) PT-INR on POD 14, (C) amount of ascites on POD 14, (D) amount of ascites on POD 30. IMAC, intramuscular adipose tissue content; T-bil, total bilirubin; POD, postoperative day; PT-INR, prothrombin-time international normalized ratio.
Risk Factors for Poor Graft Survival in Patients Undergoing LDLT
We performed univariate and multivariate cox regression analyses to examine the predictive factors for graft survival within 6 months after LDLT. Table 5 shows the results of the multivariate analysis; high donor IMAC (HR; 5.42, CI; 2.13–13.8, p = 0.0004), high MELD score (HR; 2.24, CI; 1.04–4.82, p = 0.0384), and absence of splenectomy (HR; 4.94, CI; 2.24–10.9, p = 0.0001) were independent risk factors for graft failure within 6 months.
TABLE 5
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-value | OR | 95%CI | p-value | |
| Donor variables | ||||||
| WMI | ||||||
| High (n = 132) | 1 | (References) | ||||
| Low (n = 248) | 0.71 | 0.668–2.99 | 0.3656 | |||
| IMAC | ||||||
| Low (n = 219) | 1 | (References) | 1 | (References) | ||
| High (n = 161) | 5.13 | 2.16–13.1 | 0.0003 | 5.42 | 2.13–13.8 | 0.0004 |
| Sex | ||||||
| Male (n = 235) | 1.31 | 0.592–2.89 (References) | 0.5008 | |||
| Female (n = 145) | 1 | |||||
| Age (year) | ||||||
| <50 (n = 318) | 1 | (References) | 0.1985 | 1 | (References) | 0.0514 |
| ≥50 (n = 62) | 1.75 | 0.745–4.12 | 2.46 | 0.994–6.13 | ||
| Graft | ||||||
| Right (n = 196) | 1 | (References) | 0.1885 | 1 | (References) | 0.8211 |
| Others (n = 184) | 1.66 | 0.779–3.55 | 1.1 | 0.383–2.14 | ||
| Actual GV/SLV (%) or GRWR (%) | ||||||
| ≥35 and ≥0.7 (n = 235) | 1 | (References) | 0.1781 | 1 | (References) | 0.2189 |
| <35 or <0.7 (n = 145) | 1.66 | 0.793–3.49 | 1.66 | 0.741–3.70 | ||
| ABO incompatible | ||||||
| No (n = 317) | 1 | (References) | 0.3941 | |||
| Yes (n = 63) | 0.59 | 0.179–1.97 | ||||
| Recipient variables | ||||||
| Sex | ||||||
| Male (n = 165) | 1.15 | 0.546–2.41 (References) | 0.7162 | |||
| Female (n = 215) | 1 | |||||
| Age (years) | ||||||
| <65 (n = 309) | 1 | (References) | 0.9003 | |||
| ≥65 (n = 71) | 0.94 | 0.357–2.47 | ||||
| Preoperative DM | ||||||
| No (n = 314) | 1 | (References) | 0.5752 | |||
| Yes (n = 66) | 1.29 | 0.525–3.19 | ||||
| Hepatocellular disease | ||||||
| No (n = 122) | 1 | (References) | 0.3976 | |||
| Yes (n = 258) | 0.72 | 0.338–1.54 | ||||
| HCC | ||||||
| Without HCC (n = 244) | 1 | (References) | 0.2387 | |||
| With HCC (n = 136) | 0.6 | 0.254–1.41 | ||||
| Preoperative hospital treatment | ||||||
| No (n = 252) | 1 | (References) | 0.1662 | 1 | (References) | |
| Yes (n = 127) | 0.53 | 0.214–1.30 | 0.59 | 0.236–1.46 | 0.2505 | |
| MELD score | ||||||
| ≤21 (n = 295) | 1 | (References) | 0.0114 | 1 | (References) | 0.0384 |
| >21 (n = 85) | 2.74 | 1.30–5.80 | 2.24 | 1.04–4.82 | ||
| Splenectomy | ||||||
| With splenectomy (n = 321) | 1 | (References) | <0.0001 | 1 | (References) | <0.0001 |
| Without splenectomy (n = 59) | 4.49 | 2.13–9.51 | 4.94 | 2.24–10.9 | ||
| Steatosis | ||||||
| With microvesicular steatosis (n = 23) | 1 | (References) | 0.7404 | |||
| Without steatosis (n = 163) | 0.09–5.52 | |||||
Predictors of graft loss within 6-month.
DM, diabetes mellitus; GRWR, graft recipient weight ratio; GV/SLV, graft volume/recipient standard liver volume ratio; HCC, hepatocellular carcinoma; MELD, Model for End-Stage Liver Disease.
Stratification With Predictive Factors for Graft Survival Within 6 months
Next, we examined the significance of donor IMAC for predicting graft survival. We used two risk factors excluding IMAC to stratify the patients into three groups. In the low-risk group, including patients without risk factors, and moderate-risk group, including patients with one risk factor, the graft survival rates in the high-IMAC group were significantly lower than those in the low-IMAC group (low-risk group: 94.1% vs. 98.7%, p = 0.0381; moderate-risk group: 73.1% vs. 96.2%, p = 0.0010) (Figures 6A,B). However, there was no significant difference between the high- and low-IMAC groups for the high-risk group (84.6% vs. 71.4%, p = 0.4995) (Figure 6C). We divided the patients into four groups according to the presence or absence of the three risk factors, and the graft survival rates were stratified according to the number of risk factors (0 risk factors, 98.7%; 1 risk factor, 94.8%; 2 risk factors, 75.4%; 3 risk factors, 71.4%; p < 0.0001) (Figure 6D).
FIGURE 6
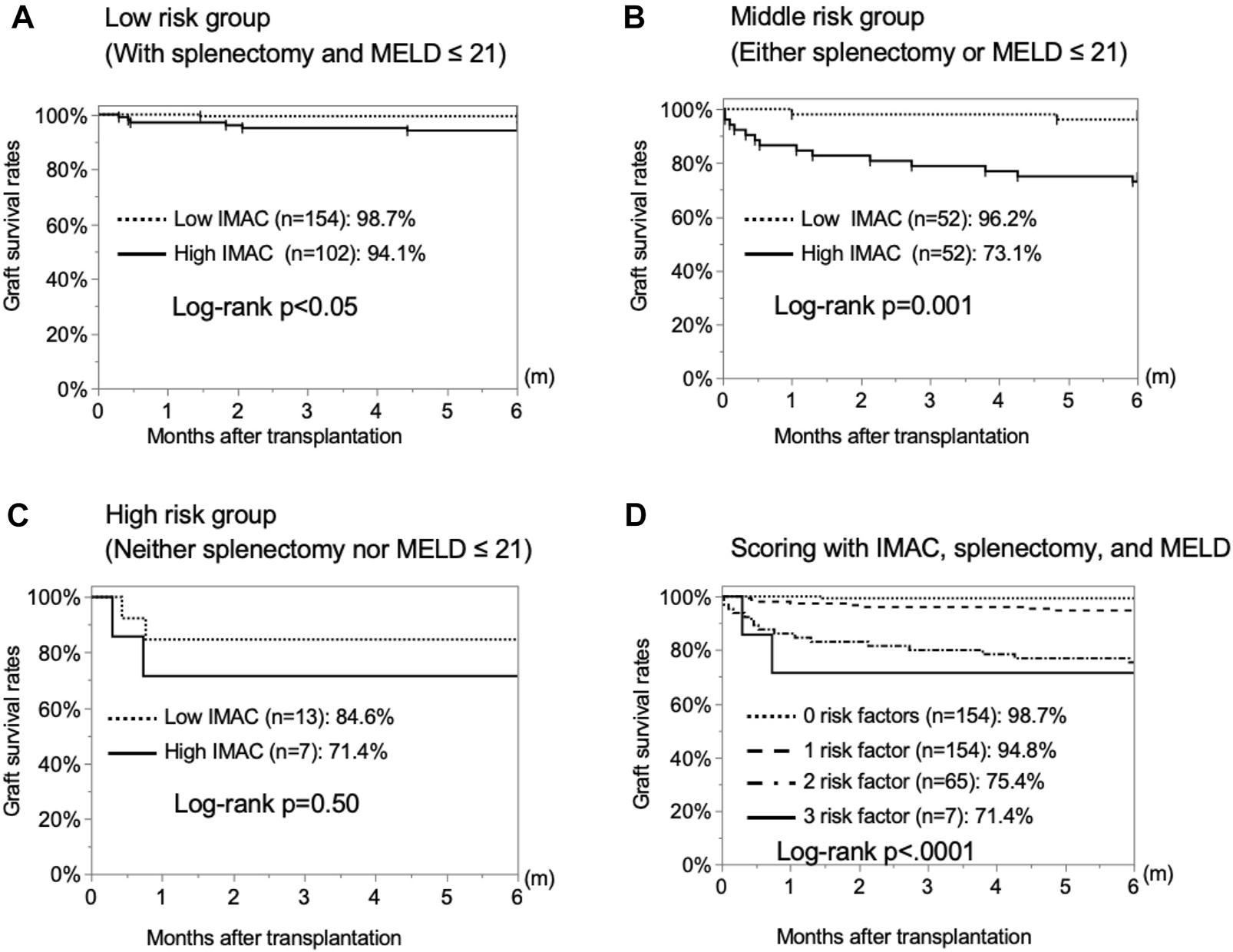
Stratification by risk factors to predict 6-month graft survival rates. The 6-month graft survival rates of recipients with high and low IMAC grafts were examined for each of two risk factors, i.e., absence of splenectomy and high MELD score. (A) No risk factors, (B) one risk factor, (C) two risk factors. (D) Stratification for 6-month graft survival rates by the number of present risk factors among the three examined ones.
Discussion
In this study, we examined the correlation of the donor SMI and IMAC with graft survival and function in LDLT. A high donor IMAC was correlated with poor graft prognosis and graft function deterioration. We stratified LDLT patients by three risk factors, including high IMAC, high MELD score, and absence of splenectomy, and found that the presence of two or more risk factors significantly reduced graft survival.
The usefulness of the IMAC for predicting the prognosis of patients with various diseases, such as cirrhosis and pancreatic cancer, has been reported, and a high IMAC was shown to correlate with poor prognosis (26, 27). In LDLT, Hamaguchi et al. firstly reported a significant association between the recipient IMAC and recipient early mortality (8). Miyachi et al. reported that combined high SMI and IMAC in male donors was an independent protective factor against graft loss after LDLT (28). To our knowledge, this is the only report showing a correlation between donor muscle quality and quantity and graft mortality. Previous studies adjusted for SMI and IMAC with donor age because there was a strong correlation between donor age and donor SMI and IMAC. In our case, there was a significant, but not strong, correlation between the donor IMAC and age. The donor selection criteria varied among institutions, and we also used donor grafts from relatively elderly donors up to 65 years of age. Donors in their 50s and 60s are expected to be relatively healthy with good muscle quality and quantity. These differences in donor selection across facilities may have affected the relationship between donor age, IMAC, and SMI, and further investigations are needed in a larger cohort. Hence, we did not adjust the IMAC for donor age, and both the male and female donor IMAC showed a strong correlation with graft survival within 6 months. In our institution, donors whose body mass index is greater than 25 or who have fatty liver in the preoperative evaluation are placed on a diet before surgery. This may have influenced the relationship between donor age and preoperative IMAC and SMI.
In LDLT, there are rarely ideal conditions in terms of recipient status and donor selection. Some compromises are often necessary in donor selection because of organ shortages. Hence, we examined under which conditions IMAC assessment is useful. In low-risk recipients, whose MELD score was 20 or lower and who could not undergo splenectomy, there was a significant difference in 6-month graft survival rates between the high-IMAC group and low-IMAC group, albeit not by a large margin. Surprisingly, in the moderate-risk group, which included patients with one risk, i.e., high MELD score or absence of splenectomy, the difference was large and significant. This may indicate that donor selection considering the IMAC may be important for moderate-risk recipients scheduled to undergo LDLT. Splenectomy is a risk factor for SFSG syndrome in LDLT, and splenectomy is recommended for recipients with small grafts or portal hypertension (19). However, splenectomy is often difficult after partial splenic embolization or spontaneous bacterial peritonitis before transplantation. Our study showed that donor selection based on the IMAC may improve graft prognosis for recipients who cannot undergo simultaneous splenectomy. Although there have been several reports on graft quality assessment in LDLT, there has been no report on the effectiveness of quality assessment markers. In the future, qualitative markers that consider other background factors should be examined. Donor age has been used as a marker for graft quality in LDLT(18). However, liver graft quality does not uniformly decline with donor age, and it is important to assess individual changes in donor grafts because there are individual differences in aging (29). In addition, the several qualitative assessment methods previously reported require liver biopsy (30, 31), while this IMAC examination is not invasive, and the IMAC can be measured with CT images obtained before surgery, with no additional burden on the donor. In our study, the IMAC was a predictive factor for graft failure, although it was correlated with donor age. This suggests that the IMAC may represent individual biological aging rather than chronological aging, and further investigations are needed.
The relationships between the musculature and liver are not well understood. Interleukin-6 (IL-6), which is implicated in both liver regeneration and metabolic functions, is secreted into the bloodstream in response to muscle contraction (32). Some epidemiological studies have reported a negative association between the amount of regular body activity and resting plasma IL-6 concentrations(33). With exercise training, IL-6 downregulation is counteracted by increased IL-6 receptor (IL-6R) expression, resulting in increased sensitivity to IL-6 (32). We hypothesized that resting plasma IL-6 concentrations are upregulated by lack of muscle use in donors with a high IMAC, and IL-6R in the liver is downregulated, thereby decreasing sensitivity and disturbing hepatocyte regeneration.
The relationship between the IMAC and graft survival was more pronounced in women. The difference may result from expression of the estrogen receptor. Estrogen is one of the most important molecular markers of liver regeneration, and it has been reported that more estrogen receptors are expressed in the male liver than in the female liver(34,35). Hence, grafts from female donors may have more directly reflected the effects of IL-6.
This study has some limitations. First, this was a retrospective and single-center study. The IMAC needs to be studied on a large scale in the future, as it is a non-invasive examination and can be evaluated using CT scans performed preoperatively. Second, there is no clear answer as to whether the IMAC should be adjusted for age. Our study did not find a strong correlation with age, while others have reported strong correlations. In any case, the IMAC is a useful prognostic marker for graft survival in LDLT, but a large-scale validation may be needed in the future to determine which IMAC or adjusted IMAC is more useful. Third, there were some significant differences in patient characteristics between the high- and low-IMAC groups. We performed univariate and multivariate analyses to more accurately examine the correlation between the IMAC and patient characteristics (Supplementary Tables S1, S2) because there were several significant differences in patient characteristics between the high- and low-IMAC groups. A high MELD score was significantly correlated with a high IMAC in female donors. Conversely, there no parameters were significantly correlated with a high IMAC in male donors. In the future, we need to assess the patient characteristics of a different, larger cohort and re-examine the usefulness of the IMAC.
In conclusion, the donor IMAC correlates with graft survival. Thus, the donor IMAC may be useful for predicting graft function and, by extension, graft mortality.
Statements
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by 2019-354, Kyushu University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conception and design: TTomiy, KTa, and TY; development of methodology: TTomiy, KTa, TK, and TTos; acquisition of data: TTomiy, TY, NH, SI, AM, KTo, YK-F, TTomin, TK, YoN, YuN, TTos, and KM; analysis and interpretation of data: TTomiy, NH, TTos, and TY; writing review and/or revision of manuscript: TTomiy, NH, and TY; study supervision: TY.
Funding
This study was supported by the following grants: AMED Grant Numbers JP20fk0310106h204 and JP20fk0210035s0503 and JSPS KAKENHI Grant Number JP18K08542. The funding sources had no role in collection, analysis or interpretation of data, or in the decision to submit the article for publication.
Acknowledgments
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2022.10723/full#supplementary-material
Abbreviations
CT, computed tomography; DM, diabetes mellitus; GV/SLV, graft volume/recipient standard liver volume; HCC, hepatocellular carcinoma; HR, hazard ratio; ICU, intensive care unit; IL-6, interleukin-6; IMAC, high intramuscular adipose tissue content; LDLT, living donor liver transplantation; MELD, Model for End-Stage Liver Disease; POD, postoperative day; PT-INR, prothrombin-time international normalized ratio; SMI, skeletal muscle mass index; T-bil, total-bilirubin.
References
1
Yugawa K Itoh S Kurihara T Yoshiya S Mano Y Takeishi K et al Skeletal Muscle Mass Predicts the Prognosis of Patients with Intrahepatic Cholangiocarcinoma. Am J Surg (2019) 218:952–8. 10.1016/j.amjsurg.2019.03.010
2
Babu R Sethi P Surendran S Dhar P Gopalakrishnan U Balakrishnan D et al A New Score to Predict Recipient Mortality from Preoperative Donor and Recipient Characteristics in Living Donor Liver Transplantation (DORMAT Score). Ann Transpl (2017) 22:499–506. 10.12659/aot.904350
3
Nakanishi R Oki E Sasaki S Hirose K Jogo T Edahiro K et al Sarcopenia Is an Independent Predictor of Complications after Colorectal Cancer Surgery. Surg Today (2018) 48:151–7. 10.1007/s00595-017-1564-0
4
Kudou K Saeki H Nakashima Y Edahiro K Korehisa S Taniguchi D et al Prognostic Significance of Sarcopenia in Patients with Esophagogastric Junction Cancer or Upper Gastric Cancer. Ann Surg Oncol (2017) 24:1804–10. 10.1245/s10434-017-5811-9
5
Uojima H Chuma M Tanaka Y Hidaka H Nakazawa T Iwabuchi S et al Skeletal Muscle Mass Influences Tolerability and Prognosis in Hepatocellular Carcinoma Patients Treated with Lenvatinib. Liver Cancer (2020) 9:193–206. 10.1159/000504604
6
Masuda T Shirabe K Ikegami T Harimoto N Yoshizumi T Soejima Y et al Sarcopenia Is a Prognostic Factor in Living Donor Liver Transplantation. Liver Transpl (2014) 20:401–7. 10.1002/lt.23811
7
Itoh S Yoshizumi T Kimura K Okabe H Harimoto N Ikegami T et al Effect of Sarcopenic Obesity on Outcomes of Living-Donor Liver Transplantation for Hepatocellular Carcinoma. Anticancer Res (2016) 36:3029–34.
8
Hamaguchi Y Kaido T Okumura S Kobayashi A Shirai H Yagi S et al Impact of Skeletal Muscle Mass Index, Intramuscular Adipose Tissue Content, and Visceral to Subcutaneous Adipose Tissue Area Ratio on Early Mortality of Living Donor Liver Transplantation. Transplantation (2017) 101:565–74. 10.1097/TP.0000000000001587
9
Severinsen MCK Pedersen BK . Muscle-organ Crosstalk: the Emerging Roles of Myokines. Endocr Rev (2020) 41:594–609. bnaa016-. 10.1210/endrev/bnaa016
10
Merli M Lattanzi B Aprile F . Sarcopenic Obesity in Fatty Liver. Curr Opin Clin Nutr Metab Care (2019) 22:185–90. 10.1097/MCO.0000000000000558
11
Kim G Kim JH . Impact of Skeletal Muscle Mass on Metabolic Health. Endocrinol Metab (2020) 35:1–6. 10.3803/EnM.2020.35.1.1
12
Nier A Huber Y Labenz C Michel M Bergheim I Schattenberg JM . Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-alcoholic Fatty Liver Disease (NAFLD). Nutrients (2020) 12:699. 10.3390/nu12030699
13
Chu MJJ Dare AJ Phillips ARJ Bartlett ASJR . Donor Hepatic Steatosis and Outcome after Liver Transplantation: a Systematic Review. J Gastrointest Surg (2015) 19:1713–24. 10.1007/s11605-015-2832-1
14
Segev DL Kucirka LM Nguyen GC Cameron AM Locke JE Simpkins CE et al Effect Modification in Liver Allografts with Prolonged Cold Ischemic Time. Am J Transpl (2008) 8:658–66. 10.1111/j.1600-6143.2007.02108.x
15
Nakamuta M Morizono S Soejima Y Yoshizumi T Aishima S Takasugi S et al Short-Term Intensive Treatment for Donors with Hepatic Steatosis in Living-Donor Liver Transplantation. Transplantation (2005) 80:608–12. 10.1097/01.tp.0000166009.77444.f3
16
Farzanegi P Dana A Ebrahimpoor Z Asadi M Azarbayjani MA . Mechanisms of Beneficial Effects of Exercise Training on Non-alcoholic Fatty Liver Disease (NAFLD): Roles of Oxidative Stress and Inflammation. Eur J Sport Sci (2019) 19:994–1003. 10.1080/17461391.2019.1571114
17
Hamaguchi Y Kaido T Okumura S Kobayashi A Shirai H Yao S et al Preoperative Visceral Adiposity and Muscularity Predict Poor Outcomes after Hepatectomy for Hepatocellular Carcinoma. Liver Cancer (2019) 8:92–109. 10.1159/000488779
18
Yoshizumi T Ikegami T Bekki Y Ninomiya M Uchiyama H Iguchi T et al Re‐evaluation of the Predictive Score for 6‐month Graft Survival in Living Donor Liver Transplantation in the Modern Era. Liver Transpl (2014) 20:323–32. 10.1002/lt.23804
19
Yoshizumi T Itoh S Shimokawa M Inokuchi S Harada N Takeishi K et al Simultaneous Splenectomy Improves Outcomes after Adult Living Donor Liver Transplantation. J Hepatol (2021) 74:372–9. 10.1016/j.jhep.2020.08.017
20
Kamath PS Wiesner RH Malinchoc M Kremers W Therneau TM Kosberg CL et al A Model to Predict Survival in Patients with End‐stage Liver Disease. Hepatology (2001) 33:464–70. 10.1053/jhep.2001.22172
21
Urata K Kawasaki S Matsunami H Hashikura Y Ikegami T Ishizone S et al Calculation of Child and Adult Standard Liver Volume for Liver Transplantation. Hepatology (1995) 21:1317–21. 10.1002/hep.1840210515
22
Yoshizumi T Ikegami T Kimura K Uchiyama H Ikeda T Shirabe K et al Selection of a Right Posterior Sector Graft for Living Donor Liver Transplantation. Liver Transpl (2014) 20:1089–96. 10.1002/lt.23924
23
Ikegami T Shirabe K Yoshiya S Yoshizumi T Ninomiya M Uchiyama H et al Bacterial Sepsis after Living Donor Liver Transplantation: The Impact of Early Enteral Nutrition. J Am Coll Surg (2012) 214:288–95. 10.1016/j.jamcollsurg.2011.12.001
24
Ikegami T Toshima T Takeishi K Soejima Y Kawanaka H Yoshizumi T et al Bloodless Splenectomy during Liver Transplantation for Terminal Liver Diseases with Portal Hypertension. J Am Coll Surg (2009) 208:e1–4. 10.1016/j.jamcollsurg.2008.10.034
25
Soejima Y Shirabe K Taketomi A Yoshizumi T Uchiyama H Ikegami T et al Left Lobe Living Donor Liver Transplantation in Adults. Am J Transpl (2012) 12:1877–85. 10.1111/j.1600-6143.2012.04022.x
26
Ebadi M Wang CW Lai JC Dasarathy S Kappus MR Dunn MA et al Poor Performance of Psoas Muscle index for Identification of Patients with Higher Waitlist Mortality Risk in Cirrhosis. J Cachexia Sarcopenia Muscle (2018) 9:1053–62. 10.1002/jcsm.12349
27
Waki Y Irino T Makuuchi R Notsu A Kamiya S Tanizawa Y et al Impact of Preoperative Skeletal Muscle Quality Measurement on Long-Term Survival after Curative Gastrectomy for Locally Advanced Gastric Cancer. World J Surg (2019) 43:3083–93. 10.1007/s00268-019-05145-1
28
Miyachi Y Kaido T Hirata M Iwamura S Yao S Shirai H et al The Combination of a Male Donor’s High Muscle Mass and Quality Is an Independent Protective Factor for Graft Loss after Living Donor Liver Transplantation. Am J Transpl (2020) 20:3401–12. 10.1111/ajt.15884
29
Tomiyama T Yamamoto T Takahama S Toshima T Itoh S Harada N et al Up‐regulated LRRN2 Expression as a Marker for Graft Quality in Living Donor Liver Transplantation. Hepatol Commun (2022) 6:2836–49. 10.1002/hep4.2033
30
Hidaka M Eguchi S Takatsuki M Soyama A Ono S Adachi T et al The Kupffer Cell Number Affects the Outcome of Living Donor Liver Transplantation from Elderly Donors. Transpl Direct (2016) 2:e94. 10.1097/TXD.0000000000000608
31
Tomiyama T Shimokawa M Harada N Toshida K Morinaga A Kosai‐Fujimoto Y et al Low Syntaxin 17 Expression in Donor Liver Is Associated with Poor Graft Prognosis in Recipients of Living Donor Liver Transplantation. Hepatol Res (2022) 52:872–81. 10.1111/hepr.13809
32
Pedersen BK Febbraio MA . Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat Rev Endocrinol (2012) 8:457–65. 10.1038/nrendo.2012.49
33
Fischer CP . Interleukin-6 in Acute Exercise and Training: what Is the Biological Relevance? - PubMed. Exerc Immunol Rev (2012) [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/17201070/ (Accessed April 5, 2022).
34
Chaturantabut S Shwartz A Evason KJ Cox AG Labella K Schepers AG et al Estrogen Activation of G-Protein–Coupled Estrogen Receptor 1 Regulates Phosphoinositide 3-Kinase and mTOR Signaling to Promote Liver Growth in Zebrafish and Proliferation of Human Hepatocytes. Gastroenterology (2019) 156:1788–804. 10.1053/j.gastro.2019.01.010
35
Yoshizumi T Shirabe K Taketomi A Uchiyama H Harada N Ijichi H et al Risk Factors that Increase Mortality after Living Donor Liver Transplantation. Transplantation (2012) 93:93–8. 10.1097/TP.0b013e318238dacd
Summary
Keywords
sarcopenia, intramuscular adipose tissue content, small-for-graft size syndrome, donor muscle status, graft quality
Citation
Tomiyama T, Harada N, Toshima T, Nakayama Y, Toshida K, Morinaga A, Kosai-Fujimoto Y, Tomino T, Kurihara T, Takeishi K, Nagao Y, Morita K, Itoh S and Yoshizumi T (2022) Donor Skeletal Muscle Quality Affects Graft Mortality After Living Donor Liver Transplantation- A Single Center, Retrospective Study. Transpl Int 35:10723. doi: 10.3389/ti.2022.10723
Received
24 June 2022
Accepted
22 November 2022
Published
09 December 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Tomiyama, Harada, Toshima, Nakayama, Toshida, Morinaga, Kosai-Fujimoto, Tomino, Kurihara, Takeishi, Nagao, Morita, Itoh and Yoshizumi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomoharu Yoshizumi, yoshizumi.tomoharu.717@med.kyushu-u.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.