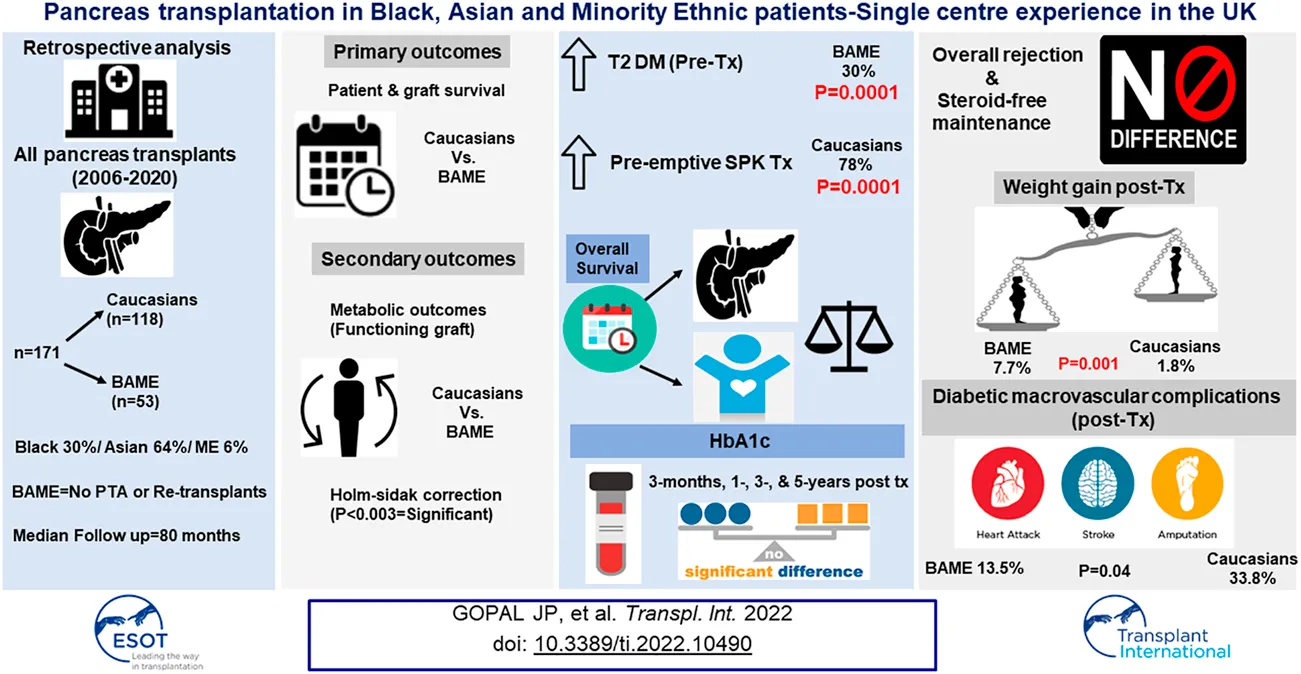
Abstract
Ethnic disparities in the outcomes after simultaneous pancreas kidney (SPK) transplantation still exist. The influence of ethnicity on the outcomes of pancreas transplantation in the UK has not been reported and hence we aimed to investigate our cohort. A retrospective analysis of all pancreas transplant recipients (n = 171; Caucasians = 118/Black Asian Ethnic Minorities, BAME = 53) from 2006 to 2020 was done. The median follow-up was 80 months. Patient & pancreas graft survival, rejection rate, steroid free maintenance rate, HbA1c, weight gain, and the incidence of secondary diabetic complications post-transplant were compared between the groups. p < 0.003 was considered significant (corrected for multiple hypothesis testing). Immunosuppression consisted of alemtuzumab induction and steroid free maintenance with tacrolimus and mycophenolate mofetil. Pancreas graft & patient survival were equivalent in both the groups. BAME recipients had a higher prevalence of type-2 diabetes mellitus pre-transplant (BAME = 30.19% vs. Caucasians = 0.85%, p < 0.0001), and waited for a similar time to transplantation once waitlisted, although pre-emptive SPK transplantation rate was higher for Caucasian recipients (Caucasians = 78.5% vs. BAME = 0.85%, p < 0.0001). Despite equivalent rejections & steroid usage, BAME recipients gained more weight (BAME = 7.7% vs. Caucasians = 1.8%, p = 0.001), but had similar HbA1c (functioning grafts) at 3-,12-, 36-, and 60-months post-transplant.
Introduction
Despite increasing interest in equitable healthcare, disparities in access to solid organ transplantation, especially for ethnic minority patients, still exists (1, 2). Most of the literature on ethnicity-based outcomes in pancreas transplantation are from the USA (3–6), and the healthcare delivery in the USA is predominantly through insurance companies. There is no equivalent data from the UK, where the healthcare system is publicly funded. In view of this and in addition, as our centre serves an ethnically diverse patient population (7) (which corresponds to the geographical location and the ethnic spread in the locality), we aimed to investigate the influence of ethnicity on the outcomes of pancreas transplantation in our patient cohort, with a special focus on metabolic outcomes. This represents the first single center experience on ethnicity-based outcomes of pancreas transplantation from the UK.
Materials and Methods
Following institutional audit committee approval, a retrospective analysis of all pancreas transplants (including simultaneous pancreas kidney-SPK, solitary pancreas, and re-transplants) performed between January 2006 and March 2020 was done. Data was collected from a prospectively maintained local database and National Health Service Blood and Transplant’s centre database.
Donor Selection Criteria
According to our center’s organ acceptance policy, all the DBD donors were less than 65 years old and all the DCD donors were less than 55 years old. The donor’s body mass index (BMI) cut off was ≤30 kg/m2. All the DCD donors had a functional warm ischemia time (systolic blood pressure <50 mmHg and/or oxygen saturation of 70%) of less than 60 min and a downtime of less than 30 min.
Immunosuppression
Immunosuppression consisted of induction with intravenous alemtuzumab 30 mg (single dose) and methylprednisolone 500 mg. Maintenance immunosuppression was with tacrolimus, mycophenolate mofetil, and a short course of steroids (7 days). Post-transplant target tacrolimus trough levels were 8–12 ng/dl.
Criteria for Transplanting Type 2 Diabetes Mellitus
Patients were classified as type 2 diabetes mellitus if they have a detectable C-peptide and the classification was predominantly based on the diagnosis made by the referring diabetologist. The following is the criteria for transplanting type 2 diabetic patients: Insulin treated diabetes along with end stage renal failure with a body mass index of ≤30 kg/m2, glycaemic lability, and insulin requirement of less than 1 Unit/Kg/day.
Outcome Measures Studied
All primary and secondary outcomes were compared between Caucasian and BAME recipients; the primary outcome measures were patient and pancreas graft survival, secondary outcome measures were metabolic outcomes among those with a functioning graft (weight gain, HbA1c, and incidence of secondary diabetic macrovascular complications post-transplant), rejection rate, and steroid usage between the two groups.
Definition of Outcome Parameters
A functioning graft is defined as remaining insulin independent post-transplantation. Secondary diabetic macrovascular complications are defined as any of the following events post-transplant: myocardial infarction, cerebrovascular accident, transient ischemic attack, and/or limb amputations (minor or major). Rejection episodes are either cellular or antibody mediated or mixed, and comprise of either pancreas or kidney rejection (in case of simultaneous pancreas-kidney transplantation). The rejections defined are either biopsy proven or those episodes that were treated based on clinical suspicion (raising serum amylase, positive circulating donor specific antibody, or delayed pancreatitis on CT scan).
Statistical Analysis
Categorical variables are expressed as frequency (%) and continuous variables as median. Differences between the categorical variables were assessed using Fisher’s exact test and differences between the continuous variables were assessed by using Mann Whitney test. Survival analysis was done by using Kaplan-Meir survival plots. Holm-Sidak correction was done for multiple comparisons and a p value of <0.003 was considered significant. All the statistical analyses were performed using Graph Pad Prism software (Version 9.0).
Results
A total of 171 pancreas transplants (SPK-129/PAK-27/PTA-4/Re-transplants-11) were performed during the study period of which 118 recipients were Caucasians and 53 were from the BAME group. Among the BAME group 64% (n = 34) were from Asian communities, 30% (n = 16) from Black communities, and 6% (n = 3) from Minority Ethnicities. The median follow-up period of the study was 80 months. The year-wise distribution of pancreas transplant activity in our center is shown in Figure 1. Donor and recipient characteristics is shown in Table 1. The HLA mismatch was grouped into 4 levels as per the NHSBT data description. The definition of each level is shown in Table 1.
FIGURE 1
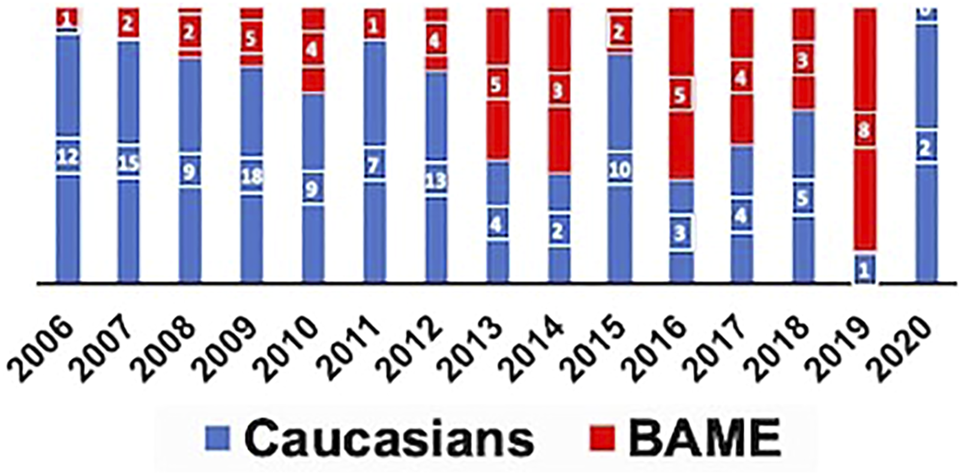
Proportion of Caucasian and BAME recipients transplanted each year.
TABLE 1
| Donor and recipient characteristics | Caucasians | BAME | p value |
|---|---|---|---|
| Donor age in years (Median) | 35 | 32 | 0.39 |
| Donor BMI in kg/sq. m (Median) | 23.10 | 24.10 | 0.12 |
| DCD donors (%) | 12.71 | 16.98 | 0.48 |
| Recipient age in years (Median) | 45 | 41 | 0.02 |
| Sensitized recipients % (CRF>5%) | 20.34 | 24.53 | 0.55 |
| Re-transplants | 11 | 0 | — |
| HbA1c at registration in mmol/mol (Median) | 71.8 | 63.9 | 0.03 |
| Duration of diabetes in years (Median) | 30.50 | 23 | <0.0001 |
| Age at onset of diabetes in years (Median) | 13 | 20 | 0.01 |
| Pre-transplant insulin use (IU/Day)-Median | 44 | 41.50 | 0.54 |
| Solitary pancreas transplants-% (PAK/PTA) | 27.12 | 13.21 | 0.05 |
| Pre-transplant type 2 diabetes (%) | 0.85 | 30.19 | 0.0001 |
| Pre-transplant secondary diabetic macrovascular complications (%) | 12.7 | 3.7 | 0.07 |
| Pre-transplant registered blind (%) | 10.17 | 13.21 | 0.55 |
| eGFR at referral in ml/min (Median) | 20 | 14.5 | 0.47 |
| Waiting time in days (Median) | 232 | 217 | 0.96 |
| Time taken for workup in days (Median) | 166 | 122 | 0.60 |
| Cold ischemia time in mins (Median) | 938 | 799 | 0.001 |
| Pre-emptive SPK transplantation (%) | 78 | 43 | 0.0001 |
| HLA group 1 (0) & 2 (0DR+0/1B) | 6% | — | 0.06 |
| HLA group 3 (0DR+2B) or (1DR+0/1B) | 28% | 32% | 0.55 |
| HLA group 4 (1DR+2B) or (2DR) | 66% | 68% | 0.83 |
Donor and recipient characteristics.
There was no pancreas transplant alone or re-transplants in the BAME cohort. The waiting time defined as the time from activation in the national transplant waiting list to transplantation were similar for both the groups, although the pre-emptive transplantation rate (for SPK transplantation) was significantly higher for Caucasian recipients. Data on workup time, which is the time from referral to our centre to activation in the national transplant waiting list, were available for 45 patients and were similar for both the groups. Data on estimated glomerular filtration rate at the time of referral for SPK transplantation were available for 51 patients and were similar for both the groups. There was a significantly higher prevalence of type 2 diabetes mellitus in the BAME group.
Patient and Pancreas Graft Survival
The 1-, 3-, and 5-year patient survival were comparable between the two groups (Table 2). There were 22 early graft losses (within 90 days post-transplant) in total. The following were the causes for early graft loss; thrombosis (n = 6), bleeding (n = 6), sudden cardiac death (n = 3), pancreas failed to perfuse on table (n = 2), severe graft pancreatitis (n = 2), duodenal anastomotic leak (n = 1), Y-graft pseudoaneurysm (n = 1), and unknown (n = 1). The early graft losses were not significantly different between the two groups (Caucasians = 16.10%, n = 19 vs. BAME = 5.6%, n = 3, p = 0.05). The 1-, 3-, and 5-year pancreas graft survival for both SPK and isolated pancreas transplants (PAK/PTA) were comparable between the two groups (Table 2).
TABLE 2
| Survival | Caucasians,% | BAME,% | Log rank p |
|---|---|---|---|
| 1-year (SPK)-Pancreas | 84.07 | 88.60 | 0.47 |
| 3-year (SPK)-Pancreas | 77.02 | 85.84 | 0.36 |
| 5-year (SPK)-Pancreas | 75.18 | 85.84 | 0.29 |
| 1-year (PAK)-Pancreas | 54.54 | 100 | 0.04 |
| 3-year (PAK)-Pancreas | 41.06 | 100 | 0.02 |
| 5-year (PAK)-Pancreas | 41.06 | 75 | 0.03 |
| 1-year (Patient) | 98.21 | 96.18 | 0.41 |
| 3-year (Patient) | 93.72 | 84.42 | 0.08 |
| 5-year (Patient) | 86.23 | 80.20 | 0.25 |
Pancreas graft and patient survival by ethnicity.
Steroid-Free Maintenance and Rejection Rate
The overall rejection rates and steroid-free maintenance rates were comparable between the two groups (Caucasians = 18.1% vs. BAME = 22.6%, p = 0.49; Caucasians = 81.8% vs. BAME = 81.1%, p = 0.92, respectively).
Metabolic Outcomes
The metabolic outcomes were compared in those recipients with a functioning graft. There were no significant differences in the HbA1c at 3-month, 1-, 3-, and 5-year post-transplant between the two groups (Figure 2). BAME recipients gained significantly more weight post-transplant compared to their Caucasian counterparts (Median percentage weight gain; BAME = 7.7% vs. Caucasians = 1.8%, p = 0.001). The overall incidence of secondary diabetic macrovascular complications post-transplant was not significantly different between the two groups (Caucasians = 33.8% vs. BAME = 13.5%, p = 0.04). There were 10 cardiovascular events (Caucasians = 8 vs. BAME = 2), 9 cerebrovascular events (Caucasians = 8 vs. BAME = 1), and 9 peripheral vascular events (Caucasians = 7 vs. BAME = 2).
FIGURE 2
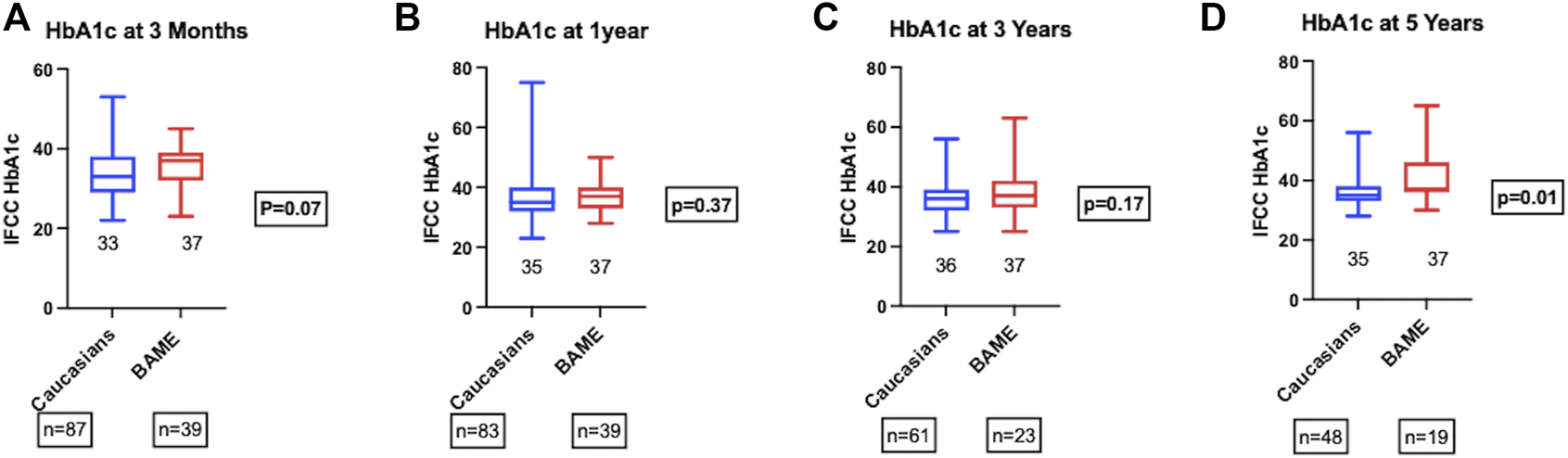
HbA1c at (A) 3-month, (B) 1-year, (C) 3-year, and (D) 5-year post-transplant.
Type 1 vs. Type 2 Diabetes-Outcomes
A subgroup analysis was performed to look into whether type 2 diabetes mellitus had an impact on the ethnicity-based outcomes. The overall pancreas graft and patient survival were similar for pre-transplant type-1 and tye-2 diabetic patients (Log rank p = 0.04, and 0.98, respectively). The post-transplant HbA1c among those with a functioning graft were similar for both type-1 and type-2 diabetic patients at 3-month, 1-, 3-, and 5-year (p = 0.02, 0.01, 0.02, and 0.02, respectively). There was no significant difference in the post-transplant median percentage weight gain either (Type-1 diabetes = 3.1% vs. Type-2 diabetes = 7.7%, p = 0.07).
Discussion
A successful pancreas transplantation leads to improvement in quality of life as well as improvement in cardiovascular risk profile, and reduction in macrovascular disease along with survival benefits in diabetic patients (8–12). Hence, the real argument for pancreas transplantation is to achieve optimal metabolic control whereas improving survival should be an added advantage. The studies reporting ethnicity-based outcomes in pancreas transplantation so far have not looked into the metabolic outcomes. As the prevalence of type-1 diabetes is less common in the non-Caucasian population (13), it is vital that metabolic outcomes should be considered alongside survival outcomes in this cohort. A review of data from the United Network for Organ Sharing (UNOS) database/Organ Procurement and Transplantation Network (OPTN) have reported that African-Americans have worse long-term survival rates (both patient and graft) compared to the other ethnic groups (3, 4). Access to pancreas transplantation has also been reported to be limited for African-Americans (5, 6), which could be due to a referral bias because of the presumed inferior outcomes in this patient group. In an era of increasing global immigration, it is crucial to avoid ethnic disparities in access to transplantation.
We noted equivalent patient and graft survival (for both SPK and solitary pancreas transplants) in Caucasian and BAME recipients. This is in contradiction to some of the major studies that have been published before (3, 4). From USA data, it transpires that Asians and Hispanics are reported to have the best survival outcomes and African-Americans have better short-term outcomes compared to Caucasians but poor longer-term outcomes (3). As Asian recipients comprised the majority of the BAME cohort in our centre, our results could be that of an Asian subtype rather than BAME community as a whole. It is also important to note that as the BMI cut off for Type 2 diabetic patients was 30 kg/sq.m, some of them might have non-classical type of diabetes rather than presumed type 2 diabetes mellitus (14). In the UK, minority groups are collectively referred to as BAME. Despite the fact that this terminology has been criticised due to the heterogeneity within the group, it is still widely in use. This heterogeneity might also explain similar survival outcomes observed in our study. Although this has been the case, our study supports the more recent observation based on data from the USA that outcomes are not necessarily inferior for non-Caucasian recipients (15–17).
HbA1c among those with a functioning graft was not significantly different between the two groups until 5 years post-transplant, although there is a trend towards higher HbA1c in BAME recipients at 5 years. Longer term follow-up data might uncover the effect of weight gain on HbA1c and graft survival. As HbA1c is known to be an independent predictor of long term pancreas graft failure (18), other metabolic parameters such as mixed meal tolerance test and C-peptide measurements were performed only selectively in patients with allograft dysfunction with consideration of intervention aimed at optimizing graft function and were not part of the routine follow up protocol.
Despite similar rejection rates and steroid usage in both the groups, BAME recipients gained significantly more weight post-transplantation. In our study we were unable to characterise whether the weight gain was due to an increase in lean body mass or due to increased adiposity. Peripheral hyperinsulinism resulting from systemic venous drainage has been postulated as a cause for excessive weight gain post-SPK transplantation (19). The explanation for higher weight gain in BAME recipients could only be speculative due to the retrospective nature of the study. Younger recipient age is known to be associated with weight gain post kidney and pancreas-kidney transplantation (20, 21). Weight gain post-transplantation has been observed in patients with a positive C-Peptide at the time of pancreas transplantation (22). The following could be the reasons for excessive weight gain in BAME group; BAME recipients were relatively younger compared to their Caucasian counterparts, and the majority of type-2 diabetics were from the BAME group. Longitudinal data on weight gain would be useful in identifying the time frame where recipients start to gain excess weight. This might help in planning dietary/behavioural modification or metabolic interventions such as the introduction of GLP-1 analogues in those at risk (23, 24). Additionally, the use of calcineurin inhibitors is known to cause insulin resistance thereby leading on to excessive weight gain (25, 26). Furthermore, it is well known that African-Americans need aggressive tacrolimus dosing to achieve optimal trough levels due to the ethnic difference in the prevalence of CYP3A5*3 genotype, which is responsible for the metabolism of tacrolimus (27, 28). Further studies looking at the circulating tacrolimus trough levels and metabolic parameters will provide more insight into strategies for optimal maintenance immunosuppression.
The incidence of pre-, and post-transplant secondary diabetic macrovascular complications were numerically higher in Caucasian recipients, although, statistically insignificant. There are several reasons for this observation. Firstly, Caucasians had an early onset and a significantly longer duration of diabetes compared to the BAME group. Hence Caucasian recipients had more macrovascular complications due to poor metabolic control. BAME recipients might have had a good metabolic control for a longer period than Caucasians before worsening control and hence the lower incidence of pre-transplant macrovascular complications. Secondly, this is also be due to a more conservative approach in listing Type 2 diabetic patients for pancreas transplantation, as is the case in BAME patients in whom Type 2 diabetes mellitus was more prevalent (29–31). Excessive weight gain observed in the BAME group could potentially lead on to post transplant metabolic syndrome and may increase the risk of cardiovascular complications in the longer term.
Prior to 2012, a majority of the patients transplanted were Caucasians and the cold ischemia times were longer. Post 2012, more BAME patients were being transplanted and the cold ischemia time was progressively shorter. Our centre’s change in practice reflected the evolving evidence base as convincing literature evidence was generated around the same time supporting pancreas transplantation in Type 2 diabetes mellitus (32–34). Due to the timeline effect there was a significant difference in the cold ischemia time between the two cohorts and there are no other explanations.
The pre-emptive transplantation rate was higher for Caucasian recipients, although, the estimated glomerular filtration rate (eGFR) at the time of referral, time taken for transplant work-up (time from referral to listing), and the waiting times were similar for both the groups. There can be several reasons for this. Most of the studies from around the world have reported that lower socio-economic status is independently associated with reduced access to pre-emptive listing (35–37). Despite a publicly funded healthcare system in the United Kingdom, socio-economic and geographic disparities in the utilisation of live donor kidney transplantation has been reported (38). Due to lack of data on socio-economic status; we are unable to comment further on that. The other reason could be the location of the patient and the proximity to a transplant centre. Being registered with a transplanting centre is known to be associated with higher pre-emptive listing because of the onsite availability of specialist services for assessing suitability. Health literacy is another important factor. Involvement of BAME ambassadors in the discussion about transplantation might reduce the socio-cultural, and language barrier and also may improve the engagement rate of BAME patients to transplantation services. The use of social media and interactive ways of reaching out, rather than traditional written pamphlets about organ donation and transplantation might also improve the awareness among BAME patients. Further multicentre studies will shed more light on BAME access to pancreas transplantation and outcomes, in addition to centre variation in practice in the UK.
This is the first study from the UK reporting ethnicity-based outcomes in pancreas transplantation and the first study reporting metabolic outcomes in Caucasian and BAME patients. Type-2 diabetes was more prevalent in BAME patients. BAME and Caucasian recipients had similar HbA1c until 5 years post-transplantation. Despite similar rejection rates and steroid usage, BAME recipients gained more weight post-transplantation. BAME patients experience similar survival outcomes (graft and patient) to those of Caucasian recipients. Although the waiting time and work-up time were similar, Caucasians had a higher proportion of pre-emptive SPK transplantation.
Statements
Data availability statement
The original contributions in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Imperial College Healthcare NHS Trust. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ARM conceptualised the study; JG collected, analysed and interpreted the data, and wrote the manuscript; AM, JC, PH, VP, FD, and ARM contributed to the data generation, made critical revisions to the manuscript and approved the final version of the manuscript.
Funding
This study received Imperial Open Access Fund from Imperial College, London for covering the article processing charge.
Acknowledgments
The authors are grateful to NHS Blood and Transplant for providing the centre data from the UK Transplant Registry.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Mathur AK Sonnenday CJ Merion RM . Race and Ethnicity in Access to and Outcomes of Liver Transplantation: A Critical Literature Review. Am J Transpl (2009) 9(12):2662–8. 10.1111/j.1600-6143.2009.02857.x
2.
Pruthi R Robb ML Oniscu GC Tomson C Bradley A Forsythe JL et al Inequity in Access to Transplantation in the United Kingdom. Clin J Am Soc Nephrol (2020) 15(6):830–42. 10.2215/CJN.11460919
3.
Brooks JT Liu R Oliver M DeLeonibus A Mei J White D et al Simultaneous Pancreas and Kidney Transplantation is Associated with Inferior Long-Term Outcomes in African Americans. Pancreas (2018) 47(1):116–21. 10.1097/MPA.0000000000000958
4.
Luan FL Kommareddi M Cibrik DM Samaniego M Ojo AO . Influence of Recipient Race on the Outcome of Simultaneous Pancreas and Kidney Transplantation. Am J Transpl (2010) 10(9):2074–81. 10.1111/j.1600-6143.2010.03211.x
5.
Melancon JK Kucirka LM Boulware LE Powe NR Locke JE Montgomery RA et al Impact of Medicare Coverage on Disparities in Access to Simultaneous Pancreas and Kidney Transplantation. Am J Transpl (2009) 9(12):2785–91. 10.1111/j.1600-6143.2009.02845.x
6.
Isaacs RB Lobo PI Nock SL Hanson JA Ojo AO Pruett TL . Racial Disparities in Access to Simultaneous Pancreas-Kidney Transplantation in the United States. Am J Kidney Dis (2000) 36:526–33. 10.1053/ajkd.2000.9793
7.
NHS Blood and Transplant. Pancreas and Islet Transplantation Annual Report 2019-2020 (2020). Available from: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/19868/nhsbt-pancreas-and-islet-transplantation-annual-report-2019-2020.pdf.
8.
Dean PG Kukla A Stegall MD Kudva YC . Pancreas Transplantation. BMJ (2017) 357:j1321. 10.1136/bmj.j1321
9.
Luan FL Miles CD Cibrik DM Ojo AO . Impact of Simultaneous Pancreas and Kidney Transplantation on Cardiovascular Risk Factors in Patients with Type 1 Diabetes Mellitus. Transplantation (2007) 84:541–4. 10.1097/01.tp.0000270617.43811.af
10.
Jukema JW Smets YFC van der Pijl JW Zwinderman AH Vliegen HW Ringers J et al Impact of Simultaneous Pancreas and Kidney Transplantation on Progression of Coronary Atherosclerosis in Patients with End-Stage Renal Failure Due to Type 1 Diabetes. Diabetes Care (2002) 25:906–11. 10.2337/diacare.25.5.906
11.
Biesenbach G Konigsrainer A Gross C Margreiter R . Progression of Macrovascular Diseases is Reduced in Type 1 Diabetic Patients after More Than 5 Years Successful Combined Pancreas-Kidney Transplantation in Comparison to Kidney Transplantation Alone. Transpl Int (2005) 18:1054–60. 10.1111/j.1432-2277.2005.00182.x
12.
Morath C Zeier M Döhler B Schmidt J Nawroth PP Opelz G . Metabolic Control Improves Long-Term Renal Allograft and Patient Survival in Type 1 Diabetes. J Am Soc Nephrol (2008) 19:1557–63. 10.1681/ASN.2007070804
13.
Norris JM Johnson RK Stene LC . Type 1 Diabetes-Early Life Origins and Changing Epidemiology. Lancet Diabetes Endocrinol (2020) 8(3):226–38. 10.1016/S2213-8587(19)30412-7
14.
Ahlqvist E Storm P Käräjämäki A Martinell M Dorkhan M Carlsson A et al Novel Subgroups of Adult-Onset Diabetes and Their Association with Outcomes: A Data-Driven Cluster Analysis of Six Variables. Lancet Diabetes Endocrinol (2018) 6(5):361–9. 10.1016/S2213-8587(18)30051-2
15.
Rogers J Jay CL Farney AC Orlando G Jacobs ML Harriman D et al Simultaneous Pancreas‐kidney Transplantation in Caucasian versus African American Patients: Does Recipient Race Influence Outcomes? Clin Transplant (2022) 36:e14599. 10.1111/ctr.14599
16.
Li Z Xiang J Liu J Wang L . Race Does Not Predict Pancreas Graft Failure after Pancreas Transplantation in the Modern Era. Clin Transplant (2022) 36:e14576. 10.1111/ctr.14576
17.
McElroy L Fridell JA . Impact of Race on Pancreas Transplant Outcomes in the Current Era: It is Not All Black and White. Clin Transplant (2022) 36(4):e14615. 10.1111/ctr.14615
18.
Davis NF Burke JP Kelly R Shields WP Kheradmand F Zimmermann A et al Predictors of 10-year Pancreas Allograft Survival after Simultaneous Pancreas and Kidney Transplantation. Pancreas (2014) 43(5):750–4. 10.1097/MPA.0000000000000119
19.
Knight RJ Islam AK Pham C Graviss EA Nguyen DT Moore LW et al Weight Gain after Simultaneous Kidney and Pancreas Transplantation. Transplantation (2020) 104(3):632–9. 10.1097/TP.0000000000002862
20.
Chang SH McDonald SP . Post-kidney Transplant Weight Change as Marker of Poor Survival Outcomes. Transplantation (2008) 85(10):1443–8. 10.1097/TP.0b013e31816f1cd3
21.
Cashion AK Hathaway DK Stanfill A Thomas F Ziebarth JD Cui Y et al Pre-transplant Predictors of One Yr Weight Gain after Kidney Transplantation. Clin Transpl (2014) 28(11):1271–8. 10.1111/ctr.12456
22.
Torabi J Rocca JP Kestenbaum E Ajaimy M DeFeo M Konicki A et al Preoperative C-Peptide Predicts Weight Gain after Pancreas Transplantation. Prog Transpl (2020) 30(2):117–24. 10.1177/1526924820913518
23.
Ramli R Gopal J Reddy M Oliver N McLean A Muthusamy A . Glucagon-like Peptide-1 (GLP-1) Agonist in Pancreas Transplant Recipients with Partial Graft Function: A Case Series. In: Diabetic Medicine, 36. Hoboken, NJ, USA: Wiley (2019). p. 14.
24.
Cariou B Bernard C Cantarovich D . Liraglutide in Whole-Pancreas Transplant Patients with Impaired Glucose Homoeostasis: A Case Series. Diabetes Metab (2015) 41(3):252–7. 10.1016/j.diabet.2014.10.004
25.
Charlton M Rinella M Patel D McCague K Heimbach J Watt K . Everolimus is Associated with Less Weight Gain Than Tacrolimus 2 Years after Liver Transplantation: Results of a Randomized Multicenter Study. Transplantation (2017) 101(12):2873–82. 10.1097/TP.0000000000001913
26.
Chakkera HA Mandarino LJ . Calcineurin Inhibition and New-Onset Diabetes Mellitus after Transplantation. Transplantation (2013) 95(5):647–52. 10.1097/TP.0b013e31826e592e
27.
Taber DJ Gebregziabher MG Srinivas TR Chavin KD Baliga PK Egede LE . African-American Race Modifies the Influence of Tacrolimus Concentrations on Acute Rejection and Toxicity in Kidney Transplant Recipients. Pharmacotherapy (2015) 35(6):569–77. 10.1002/phar.1591
28.
MacPhee IAM Holt DW . A Pharmacogenetic Strategy for Immunosuppression Based on the CYP3A5 Genotype. Transplantation (2008) 85(2):163–5. 10.1097/TP.0b013e3181609054
29.
Singh RP Rogers J Farney AC Hartmann EL Reeves-Daniel A Doares W et al Do pretransplant C-Peptide Levels Influence Outcomes in Simultaneous Kidney-Pancreas Transplantation? Transplant Proc (2008) 40:510–2. 10.1016/j.transproceed.2008.01.048
30.
Friedman AL Friedman EA . Pancreas Transplantation for Type 2 Diabetes at U.S. Transplant Centers. Diabetes Care (2002) 25:1896. 10.2337/diacare.25.10.1896
31.
Ciancio G Burke GW . Type 2 Diabetes: Is Pancreas Transplantation an Option?Curr Diab Rep (2014) 14(11):542. 10.1007/s11892-014-0542-9
32.
Orlando G Stratta RJ Light J . Pancreas Transplantation for Type 2 Diabetes Mellitus. Curr Opin Organ Transpl (2011) 16(1):110–5. 10.1097/MOT.0b013e3283424d1f
33.
Light JA Barhyte DY . Simultaneous Pancreas-Kidney Transplants in Type I and Type II Diabetic Patients with End-Stage Renal Disease: Similar 10-year Outcomes. Transplant Proc (2005) 37(2):1283–4. 10.1016/j.transproceed.2004.12.215
34.
Light JA Sasaki TM Currier CB Barhyte DY . Successful Long-Term Kidney-Pancreas Transplants Regardless of C-Peptide Status or Race. Transplantation (2001) 71(1):152–3. 10.1097/00007890-200101150-00025
35.
Grams ME Chen BP Coresh J Segev DL . Preemptive Deceased Donor Kidney Transplantation: Considerations of Equity and Utility. Clin J Am Soc Nephrol (2013) 8:575–82. 10.2215/CJN.05310512
36.
Riffaut N Lobbedez T Hazzan M Bertrand D Westeel PF Launoy G et al Access to Preemptive Registration on the Waiting List for Renal Transplantation: A Hierarchical Modeling Approach. Transpl Int (2015) 28:1066–73. 10.1111/tri.12592
37.
van Dellen D Burnapp L Citterio F Mamode N Moorlock G van Assche K et al Pre-emptive Live Donor Kidney Transplantation-Moving Barriers to Opportunities: An Ethical, Legal and Psychological Aspects of Organ Transplantation View. World J Transpl (2021) 11(4):88–98. 10.5500/wjt.v11.i4.88
38.
Wu DA Robb ML Watson CJE Forsythe JLR Tomson CRV Cairns J et al Barriers to Living Donor Kidney Transplantation in the United Kingdom: A National Observational Study. Nephrol Dial Transpl (2017) 32(5):890–900. 10.1093/ndt/gfx036
Summary
Keywords
pancreas transplantation, race, ethnicity, metabolic outcomes, SPK transplantation, Caucasian, BAME
Citation
Gopal JP, McLean A, Crane J, Herbert P, Papalois V, Dor FJMF and Muthusamy AR (2022) Pancreas Transplantation in Black, Asian and Minority Ethnic Patients-Single Centre Experience in the UK. Transpl Int 35:10490. doi: 10.3389/ti.2022.10490
Received
08 March 2022
Accepted
16 May 2022
Published
15 June 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Gopal, McLean, Crane, Herbert, Papalois, Dor and Muthusamy.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anand Rathnasamy Muthusamy, anand.muthusamy@nhs.net
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.