Abstract
The effectiveness of liver transplantation to cure numerous diseases, alleviate suffering, and improve patient survival has led to an ever increasing demand. Improvements in preoperative management, surgical technique, and postoperative care have allowed increasingly complicated and high-risk patients to be safely transplanted. As a result, many patients are safely transplanted in the modern era that would have been considered untransplantable in times gone by. Despite this, more gains are possible as the science behind transplantation is increasingly understood. Normothermic machine perfusion of liver grafts builds on these gains further by increasing the safe use of grafts with suboptimal features, through objective assessment of both hepatocyte and cholangiocyte function. This technology can minimize cold ischemia, but prolong total preservation time, with particular benefits for suboptimal grafts and surgically challenging recipients. In addition to more physiological and favorable preservation conditions for grafts with risk factors for poor outcome, the extended preservation time benefits operative logistics by allowing a careful explant and complicated vascular reconstruction when presented with challenging surgical scenarios. This technology represents a significant advancement in graft preservation techniques and the transplant community must continue to incorporate this technology to ensure the benefits of liver transplant are maximized.
Introduction
Since the introduction of liver transplant as a treatment option for end stage liver disease and liver cancer in the 1960s, its role and potential benefits have expanded substantially (1, 2). In the initial phase of liver transplantation following the acceptance of the brain death concept (3), graft options were restricted to whole livers from deceased donors. In the modern day, this has expanded to include reduced size, auxiliary, domino, and split grafts from either deceased or living donors. The utilization of these different graft types has partly been driven by the fact that the accepted indications for liver transplant have also expanded over the last half century (4–6). Benefits from transplantation have been demonstrated in situations other than cirrhosis or primary liver cancer, including metabolic disease, colorectal liver metastases, and perihilar cholangiocarcinoma (6–8). Demand for organs continues to increase and exceeds the supply (9). Therefore, a proportion of patients on the waitlist miss their window of opportunity and are delisted. As an example, during 2018 in the United States (US), 18.6% (equating to 1471 patients) of the year’s starting waitlist was removed due to either death or becoming too sick to transplant (9). Maximizing graft utility is therefore paramount, and proven strategies, such as normothermic machine perfusion (NMP), should be routinely incorporated into clinical practice for certain graft-recipient scenarios.
The assessment that a patient, with an accepted indication, can be transplanted with a good outcome is reliant on social, psychological, medical, and surgical factors. Further to this, the risk - benefit assessment must be individualized as not all perspective recipients present with the same risks, nor should they all be expected to glean the same benefits. Despite research into overall risk (10, 11), the transplantability of each patient is largely subjective. Surgically high-risk recipients are those patients with certain factors that present additional obstacles, requiring the surgical team to adapt their strategy from that of a routine transplant. These may include extensive portal vein thrombosis, previous hepatobiliary surgery, or previous liver transplantation. In these situations, altered anatomy, obliterated tissue planes, and excessive bleeding may be encountered. There may also be the requirement for complex vascular reconstruction and flow modulation techniques for both the artery and portal vein. In combination, these factors increase the surgical insult, prolong the cold ischemic time (CIT) of a traditionally preserved liver graft waiting to be implanted, and may exacerbate the ischemia-reperfusion injury process. This may result in physiological instability and poor outcomes, especially if accompanied by early graft dysfunction. Historically, graft options for surgically high-risk patients were restricted to only those of optimal quality as they could withstand the longer cold ischemic period and provide immediate graft function. NMP can minimize CIT, objectively assess graft function, and safely prolong the preservation time (12, 13). We have recently published the results of our approach for retransplant candidates, which utilizes NMP for suboptimal grafts (14). In addition to facilitating the use of “orphan” grafts for a group that were disadvantaged by the current allocation scheme, it provides logistical benefits for the completion of a difficult operation (Figure 1) (14). The additional challenges imparted by the COVID-19 pandemic and the consequential recipient testing protocols and strain on intensive care resources has meant that our institution has been required to increasingly apply NMP for non-patient-related reasons. Although beneficial via other means, other machine perfusion techniques such as normothermic regional perfusion and hypothermic oxygenated machine perfusion do not offer the same advantage in operative logistics (15, 16).
FIGURE 1
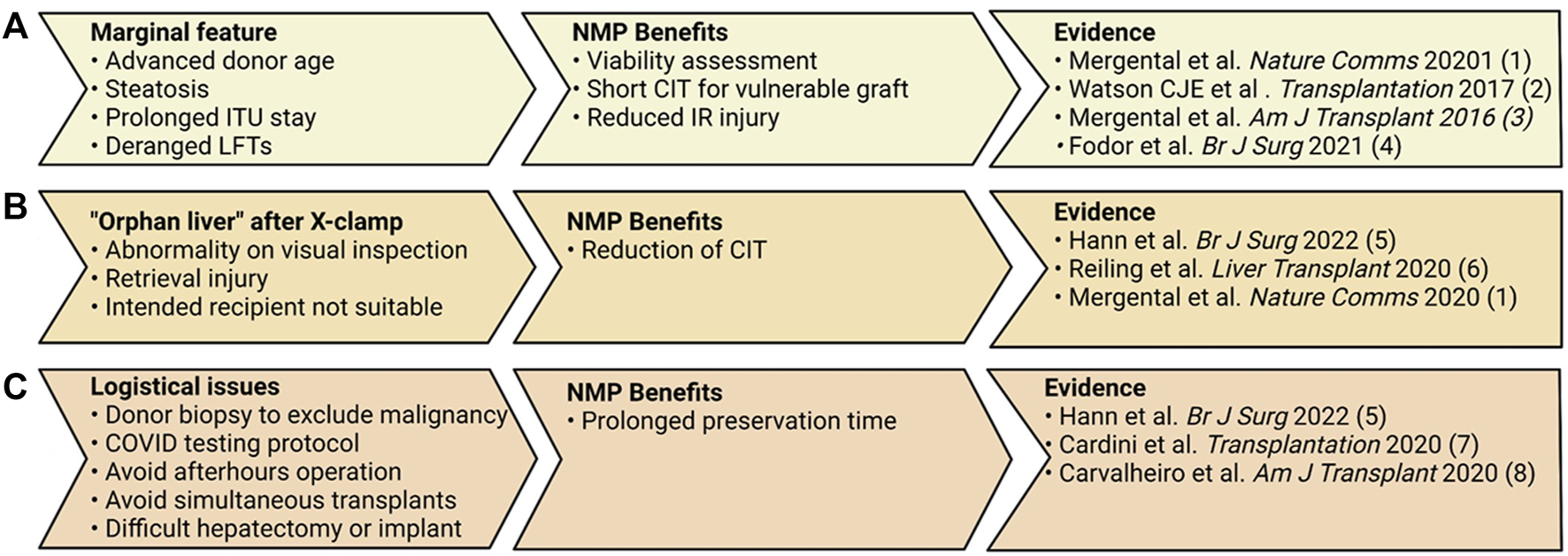
Three scenarios in which normothermic machine perfusion (NMP) can applied effectively by a transplant center. (A) In the setting of marginal graft features, NMP can shorten the CIT these grafts are subjected to and objectively assess the grafts’ function. (B) NMP is ideal for the situation in which an offer is received and CIT has already commenced, or one recipient is not deemed appropriate and more time is required to prepare another recipient. (C) Lengthening the preservation time can offer numerous benefits for the logistics around the transplant operation (12–14, 27, 30, 33, 40, 43).
Selecting donors and organs that can be safely used in the transplantation process is a challenging task. Organs chosen for transplantation should pose a minimal risk of donor-derived infection or malignancy, and ideally function in both the short and longer term. Graft-recipient matching goes beyond the size and ABO blood type compatibility, it relies on clinical judgement of the donor organ and its expected graft function, with the recipient stability to withstand the physiological disturbance caused by ischemia reperfusion injury (17). The majority of western countries, including the United Kingdom (UK), rely on deceased donors as the source of liver grafts and therefore operate with a limited supply of organs. This means difficult decisions need to be made regarding allocation of grafts (18). On the other hand, numerous deceased donor liver grafts are declined for transplantation each year, undoubtedly a portion of these would pose a significant risk to the recipient and truly are unsuitable (9). However, a proportion of these grafts may be suitable for transplantation with the use of machine preservation strategies such as NMP. In this article, we will further outline the reasons for using this preservation technology for high-risk recipients, and how NMP can assist in graft assessment.
High Surgical Risk Recipients
A liver transplant is a challenging operation, with numerous risks due to both the technical aspects of the procedure and the recipients’ physiology. Despite advances in technique, such as preservation of the recipient inferior vena cava (2, 19) and reduction in perioperative blood loss, the surgical insult remains significant. Beyond the standard recipient with either acute liver failure or chronic end stage liver disease, additional technical factors may increase the challenge and risk for the surgeon and recipient, respectively. Previous hepatectomy or liver transplant, vascularized adhesions due to portal hypertension, and displacement of anatomical structures that all result in unclear dissection planes are such examples. These factors lengthen the hepatectomy phase and prolong the CIT of a graft if preserved via SCS. Endovascular stents and thrombi in major vascular structures or even extending into the right portion of the heart may require extensive logistics for use of a veno-venous bypass, and involvement of cardiothoracic teams. The ability of NMP to abbreviate the cold ischemic period but prolong the graft preservation in a safe manner provides a mechanism to expand graft options for these patients, accommodate the complex logistics, and mitigate the risk of unanticipated surgical factors.
It must be emphasized that with the combination of NMP, a high risk recipient, and a suboptimal graft, the implantation time must be kept short as the graft is already warm and therefore ATP depletion will proceed at a faster rate than following SCS. Scientific evidence for this is yet to be published, however more prolonged implantation times with NMP-preserved grafts are likely unfavorable. Alternative surgical strategies can be used in complex scenarios with NMP as opposed to cold storage. Rather than striving for a short cold ischemic period with the standard preservation method and early reperfusion of the graft, NMP allows time for a careful dissection to gain proper vascular control prior to the implantation phase and this is particularly useful in the setting of portal vein or late hepatic artery thrombosis when the implantation can be complicated (20). The length of this period has previously been shown to correlate with early graft function and long term outcome, with suboptimal grafts being impacted the most (21, 22). A portal cavernoma, fragile varices, and large spontaneous porto-systemic shunts may have developed following thrombosis of the portal vein. Retransplantation in the setting of late hepatic artery thrombosis is indicated when refractory or recurrent biliary sepsis occurs, often accompanied by inflamed tissues and infected bilomas. These obstacles must be overcome, and the implantation technique of the graft must ensure adequate inflow to the graft for a successful outcome (23). In the setting of late hepatic artery thrombosis, Buccholz et al. reported that aortic conduits were required in 83% of recipients (24). Regardless of whether this originates from a supraceliac or infrarenal position, the arterialization time reported in a multicenter study can be lengthy (23). The presence of a portal vein thrombus may necessitate extensive dissection in a caudad direction posterior to the pancreas to the splenic and superior mesenteric vein confluence, and a venous interposition graft if the native portal vein cannot be adequately thrombectomized. In an effort to keep the implantation and arterialization time to a minimum, we ensure the arterial and/or venous conduit is anastomosed on the proximal (recipient) side prior to disconnecting the graft from NMP and commencing the implantation.
Appropriate graft-recipient matching has been shown to be an important factor in ensuring optimal liver transplant outcomes, with marginal grafts generally preferred for recipients with lower model for end stage liver (MELD) scores (25). The rationale for this is that these recipients have a greater physiological reserve to tolerate an early period of graft dysfunction. Therefore, the suggestion that high-risk and complex recipients should be transplanted with marginal grafts contradicts the common belief that a premium quality liver graft will be required in these scenarios (26). This belief likely stems from many decades of experience with static cold storage in which the CIT was prolonged, resulting in more severe reperfusion injury and graft dysfunction as a consequence. The introduction of NMP has challenged this belief, with several successful reports of using suboptimal or “orphan” grafts for challenging cases (12, 14, 27–30). In a recent study from our center that compared a prospective group undergoing late liver retransplantation with suboptimal NMP-preserved grafts with two retrospective cold storage control groups, the outcomes were shown to be equivalent (14). Extrapolating the results from the high-risk group in this study, several of whom were undergoing a third graft, this approach can be employed for others that are complex from a surgical perspective. The utilization of NMP can therefore expand the proportion of grafts suitable for this high-risk group.
Graft Assessment Normothermic Machine Perfusion
Assessment of graft viability via ex situ machine perfusion remains an imperfect science, with many unanswered questions (31). Many different institutions have reported the criteria they apply to determine if a graft is viable, and these are being constantly altered to reflect the growing experience (12, 13, 28, 32–34). These have been extensively reviewed by other authors (35–37). The different markers reportedly used for graft assessment during NMP can be considered as surrogate indicators of graft injury and hepatocellular or cholangiocyte function (Figure 2). Graft injury sustained prior to NMP and poor hepatocellular function will manifest as severe graft dysfunction in the early post-operative period (38). Cholangiocyte injury sustained during the preservation process may be detectable through altered bile composition, and manifest as ischemic type biliary lesions (ITBLs). Although there is growing evidence that NMP can reduce the incidence of ITBLs, the risk of this complication is undoubtedly higher in DCD grafts (13, 39, 40).
FIGURE 2
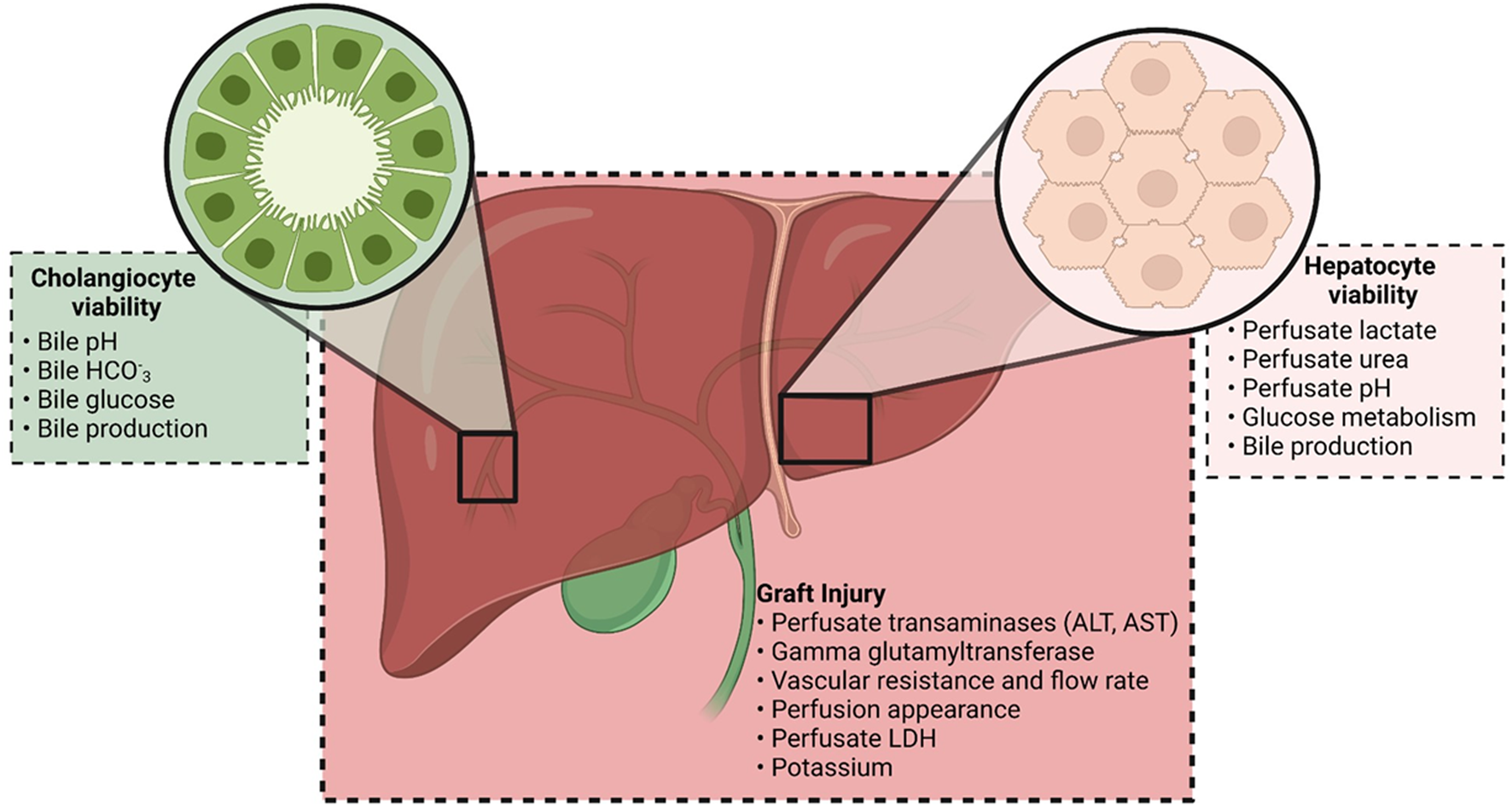
Markers currently being used by transplant centers to assess viability on the NMP circuit. Markers can be considered as indicating graft injury and cholangiocyte or hepatocyte viability. The combination of these markers is used in the viability criteria reported by different institutions.
Lactate clearance is the one viability marker that is consistent across all reported liver NMP viability criteria. Prior to connection of the liver to the NMP circuit which contains preserved human red blood cells, the lactate is usually high (10–20 mmol/L) due to the high concentrations of this substance within preserved bags of human erythrocytes (41). Following connection of a liver with functional hepatocytes, lactate is converted to pyruvate by lactate dehydrogenase in the hepatocyte cytosol (42). This lactate uptake and metabolism has been reported to occur predominantly in zone 1 hepatocytes which receive the best perfusion and oxygenation, therefore it has been proposed that a failure to clear lactate indicates an extensive and panlobular injury (35). Despite this, adequate lactate clearance and post operative graft function have been demonstrated in the setting of extensive hepatocyte necrosis (29). The normal pattern of lactate clearance on NMP is a rapid early phase, that slowly tapers off and reaches a plateau for the remaining period of perfusion (33, 43). This early change in lactate concentration is clinically useful as it gives an early indication of the likelihood the graft is transplantable as other indicators such as bile production and glucose metabolism generally become evident later. It has previously been demonstrated that some transplantable grafts do not follow the above “normal” pattern (28). Instead, they may demonstrate a slower but gradual decline or a slight increase in lactate concentration following the initial rapid decline (28). In these situations, we recommend continuing the duration of the perfusion to determine if the ability to clear lactate and maintain it at ≤2.5 mmol/L is possible. In our experience with using a closed circuit device (Organox®, Oxford, United Kingdom), we have noted that a rise in lactate (after previously rapid clearance) is often the result of bleeding from the liver hilum and this should be addressed. It may occur due to a larger proportion of blood returning directly to the reservoir bag, and therefore bypassing the metabolism and clearance by the hepatocyte. We apply the previously reported viability criteria regardless of the recipient, and also have reported successful outcomes recently with further expansion of the time cut-off for DBD grafts (13, 28).
Expanding the Pool of Transplantable Grafts
Optimizing the safe utilization of deceased donor livers should be an ambition of all transplant programs. Despite recent improvements, the organ discard rate for retrieved organs from deceased donors in the US and UK is 8.4% and 18.2%, respectively (9, 44). Previous work has revealed concern regarding organ quality or donor history which accounts for the majority of transplant center declines (45, 46). Additional reasons for organ decline that emerge at the time of retrieval include the macroscopic appearance, a prolonged donor warm ischemic time in the setting of DCD donation, and unexpected laparotomy findings suggestive of malignancy. Despite sub-optimal donor features, many centers have reported acceptable results using livers “that nobody wants”, otherwise known as “orphan” livers (13, 27, 45, 46). This is a real-world demonstration that there is capacity to increase the “standard” acceptance criteria of liver grafts and NMP is a safe way to continue expanding these boundaries.
Donor Medical History
The only absolute contraindications to transplanting a deceased donor’s liver based on their medical history is an established diagnosis of cirrhosis, primary central nervous system lymphoma, hematological or metastatic malignancy, active JC viral infection, or a transmissible spongiform encephalopathy (47, 48). The more common reasons in the current era for graft decline include donor age, alcohol history, abnormal liver function test results, and peri-mortem events (49, 50). Separating out the proportion of these grafts that can be safely transplanted is a challenging task, and we find NMP technology beneficial.
Donor Age
The physiological effects of age have less of an impact on the liver compared with the kidney and heart. However, both structural and functional differences exist in livers of older donors. The metabolic function of the hepatocyte has been shown to be decreased in the elderly. Peterson et al. demonstrated that the phosphorylation and oxidative capacity of mitochondria within the hepatocytes of the elderly (61–84 years) was reduced by 40% compared with young controls (18–39 years) (51). This was also accompanied by an increase in the triglyceride content of hepatocytes, which is further increased with an insulin-resistant state (51, 52). These deficiencies make the graft of an older donor less tolerant of the ischemic periods inherent in the transplant process. Structural changes that have been described in the livers of elderly people include increased fibrosis, possibly as the result of enhanced Th2 cytokine expression from macrophages (53).
The threshold for considering a donor to be of advanced age varies considerably, ranging from ≥40 to ≥80 years of age (54–56). Recent evidence demonstrates that elderly donors of DCD (>70 years) and DBD (>80 years) grafts can be used safely with 5 years graft survival rates of 74% and 77%, respectively, and therefore advanced age should not be a contraindication (54, 57). Grafts from older donors should be viewed as more susceptible to cold preservation injury for the aforementioned reason, therefore every effort should be made to minimize the CIT and NMP is an ideal strategy. Through the application of NMP on receipt of the graft at the recipient center, even recipients that will likely require a prolonged hepatectomy can be transplanted with a graft from an elderly donor with a short CIT.
Alcohol History
Alcohol-induced liver damage progresses in severity from hepatic steatosis to alcoholic steatohepatitis and then cirrhosis (58). Approximately 90% of individuals who consume excessive quantities of alcohol for at least 2 weeks, will develop macrovesicular hepatic steatosis (58). However, this will resolve following even a short period of abstinence but approximately one third of individuals with hepatic steatosis from alcohol will progress to steatohepatitis (58). Both the transaminases and gamma-glutamyl transferase (GGT) are relevant as they may give an indication of acute hepatocyte injury and steatosis, respectively (59).
The literature describing the transplant outcomes of donors with an excessive alcohol history is limited. Mangus et al. reported similar short and medium-term outcomes in groups of recipients that received a graft from either a donor with or without a history of excessive alcohol consumption (60). The graft recipients peak alanine aminotransferase (ALT) was higher in the excessive alcohol group, however the incidence of graft loss within the first 90 days was similar (6% vs. 7%, p = 0.75) (60). These authors analyzed the histological evidence provided from the post reperfusion biopsies of the excessive alcohol consumption group, and only 8% had fibrosis (any severity) and 9% had steatosis (>20%), respectively (60). However these authors defined excessive alcohol consumption as ≥2 more alcoholic drinks per day on a chronic basis (“at least several years”) (60). This may mean that these findings underestimate the risk in donors who may consume in excess of 10 alcoholic drinks per day, which is not uncommon in the United Kingdom. At our institution, donors with a history of excessive alcohol consumption are given full consideration if there is no established diagnosis of alcoholic liver disease. Visual inspection of the graft occurs at the time of retrieval and if concerns regarding significant steatosis or steatohepatitis exist, a graft biopsy is performed and our experienced liver histopathologists review the frozen sections before proceeding to transplantation. If the donor (and retrieval procedure) are occurring at a distant hospital, utilizing NMP provides additional time for histological and functional assessment.
Liver Function Tests
The commonly described “liver function tests”, actually provide minimal insight into the liver’s synthetic function, if at all. Despite this, even minimal elevations of liver enzymes are considered as criteria for defining marginal donors as per Eurotransplant criteria (61, 62). Alanine aminotransferase (ALT) is found predominantly in the cytoplasm of liver and kidney cells, whereas AST is found in both the cytoplasm and mitochondria of all cells (63). Therefore sources of elevation outside the liver, such as skeletal muscle damage and hemolysis, should be considered when considering a potential donor’s transaminases (63). In addition, factors such as hemodynamic instability, trauma, and sepsis may result in deranged liver function results through various mechanisms (50, 59, 64). Mangus et al. reported comparable outcomes in patients transplanted with grafts from donors with elevated peak ALT levels >1000 IU/L and 500–1000 IU/L when compared to those with peak ALT <500 IU/L. In the group with elevated peak ALT levels (≥500 IU/L), anoxia was the cause of death in a significantly higher proportion of patients compared to those with peak ALT <500 IU/L. Therefore, the authors concluded that an acute anoxic event likely accounted for the liver function derangement (50). Interestingly, the extent of necrosis on graft biopsy did not positively correlate with the peak ALT elevation (50). Adding to the argument that these type of donors are an acceptable way to expand the pool, data from the Scientific Registry of Transplant Recipients (US) demonstrate the discard rate being approximately 70% for a donor’s high transaminases (>1000 IU/L) as opposed to 22% for low transaminases (<1000 IU/L) (65).
The viability assessment provided by NMP is the ideal preservation platform for these grafts in the three aforementioned clinical scenarios that lead to organ discard, and has previously been shown to be effective even when the transaminase elevation is accompanied by significant hepatocyte necrosis (29). Our institution gives full consideration to the utilization of livers from donors with deranged transaminases, and places a greater importance on the trend rather than the peak level. The half life of AST and ALT in the human circulation differs, with it being approximately 17 (±5) h for AST and 47 (±10) h for ALT (63). Therefore following any transient insult to the liver during the terminal illness, it should be expected that the peak AST will be higher, occur earlier, and more rapidly decline than ALT, and therefore a decline in donor AST will be the first sign of a resolving insult. Donor bilirubin is undoubtedly an important biochemical test in the context of donor evaluation and is more useful in identifying donors that likely have a poorly functioning liver than transaminases. In support of this belief, it was the only biochemical variable identified as having a significant relationship to transplant outcome in the construction of a UK donor liver quality model by Collet et al. (66). In the absence of an extrahepatic cause of donor hyperbilirubinemia, our upper limit of acceptance for donor bilirubin is 2 x the upper limit of normal (40 μmol/L, 2.4 mg/dl). In the series of 14 donors with transaminases >1000 IU/L reported by Martins et al., only one had a peak bilirubin above this value. Other causes of jaundice in the donor such as hemolysis or Gilbert syndrome (UDP glucoronosyltransferase-1A1 deficiency) should be considered, and useful additional tests such as lactate dehydrogenase and conjugated and unconjugated bilirubin should be requested.
Peri-Mortem Events
In the context of deceased organ donation, consideration of the events and pathological process that resulted in brain death (or the consideration for DCD donation) is required. Consensus guidelines are available for the scenario of donor malignancy or infection and are beyond the scope of this review (47, 48). In a seminal publication by Feng et al. using US registry data, which reported the donor risk index (DRI), death from trauma or hypoxic brain injury (HBI) was associated with better outcomes than cerebrovascular accident or other causes (55). However, this finding was not replicated with UK data in derivation of the donor liver index (DLI) (66). Despite these statistical models taking into account several donor and graft features, no significant difference exists between those that satisfied our institution’s viability criteria and those that did not (Figure 3).
FIGURE 3
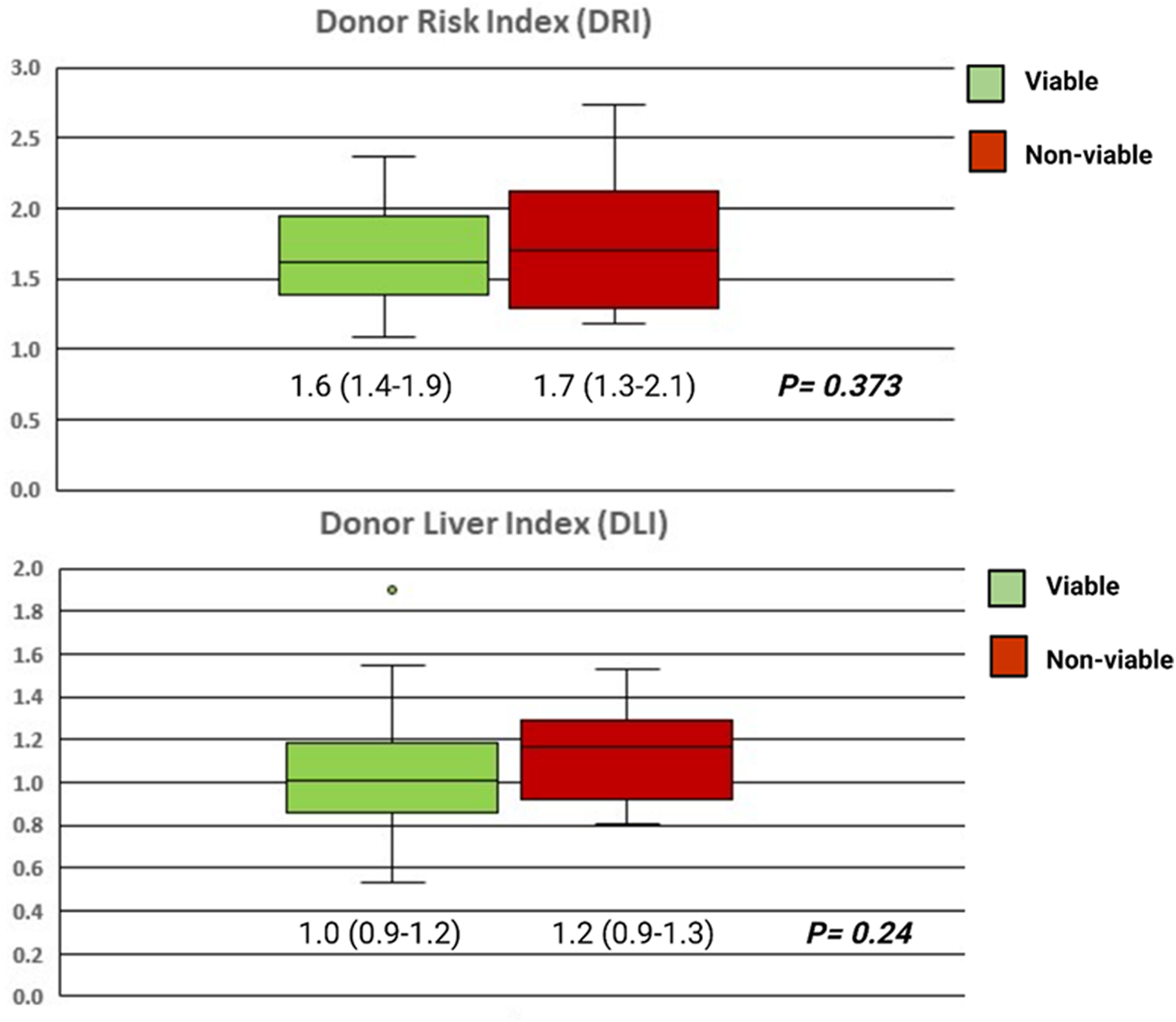
Box plots showing the DRI and DLI of the grafts that were assessed as viable and transplanted following NMP (green, n = 95), and those that were assessed as non-viable (red, n = 12). Groups compared with the Mann-Whitney U test and independent samples T-test for the DRI and DLI, respectively.
A donor ICU length of stay >7 days is considered an extended criteria donor by Eurotransplant, whereas previous consensus meetings have stipulated an ICU length of stay <5 days is ideal for DCD donors (59, 62). It seems that a consensus on what comprises a prolonged donor ICU stay is yet to be reached (67). A longer ICU stay does appear to be associated with increased donor infections, however a positive culture in the donor does not appear to influence the recipient’s outcome (67, 68). In a study by Misar et al., which compared the outcomes of pediatric recipients who received a graft from a donor following a short (<5 days) or long (≥5 days) ICU stay, there was no difference in overall graft or patient survival (67). In our practice, we agree with the notion put forward by Strasberg et al. in which it is not the length of ICU stay per se that is deleterious, rather the events that transpire during this period (69). These could include hypotensive episodes, sepsis, exposure to hepatotoxic medications, surgical procedures, and lack of adequate nutritional support.
A blunt traumatic injury may result in serious intracranial injuries and brain death as a result. There may also be an associated traumatic liver injury, which is most commonly lacerations or contusions of the liver parenchyma (70). These traumatically injured livers may still be transplantable and certain strategies, including a back table graft reduction, have been reported with success (70). A concern with utilizing NMP in this scenario is that uncontrollable bleeding may occur on the perfusion circuit due to the high concentration of heparin and lack of coagulation factors. In general, we have seldom had problems with bleeding on the NMP machine from traumatic injuries sustained prior to or at the time of organ retrieval. Application of gauze swabs and hemostatic products have been used to control minor bleeding from capsular damage.
Graft Steatosis
A frequent reason for a graft to be assessed as suboptimal and discarded is the presence of steatosis (71). Although the cause of hepatic steatosis is likely multifactorial, there is a strong association with body mass index (BMI) (72). This presents a significant issue for the field of liver transplantation as the western population is becoming more obese. Therefore, transplant clinicians must aim to better understand the implications and limitations of steatotic livers and further develop effective medical and surgical strategies to facilitate their safe utilization.
Multiple studies have demonstrated that even severe microsteatosis does not have a deleterious effect on transplant outcome (73–75). It is the extent of macrovesicular steatosis which is the main concern of the transplant surgeon, as an association with primary non-function and reduced graft survival has been demonstrated in the past (76, 77). Despite graft biopsy and fresh frozen histological assessment being considered the gold standard (71), the method (visual inspection or histology) used to assess graft steatosis is variable. As an example, the UK organ donation service does not have pathology services available after-hours and therefore retrieval surgeons rely on visual inspection and palpation. Furthermore, the implanting surgeon is limited to photographic assessment alone. A limitation of visual assessment and palpation of graft texture is the inability to reliably distinguish microvesicular from macrovesicular change. In a publication by Yersiz et al. that compared surgeons’ visual assessment to pathologists’ microscopic assessment, the positive predictive value of a surgeons’ assessment of >30% macrovesicular steatosis as per histology was only 52% (78). It must also be noted that histological assessment also has its own limitations including interpretation differences between pathologists (79).
The extent of macrosteatosis that represents a graft that is totally unusable remains unclear (80). Studies have frequently failed to define the type of steatosis, making it difficult to draw conclusions draw given the aforementioned differing implications (79, 80). Nevertheless, it is well known by liver transplant clinicians that recipients of livers with moderate to severe steatosis have a more turbulent early post-operative period. In general, more than 30% of macrosteatosis is viewed as increasing the risk of clinically relevant early graft dysfunction. Safely utilizing steatotic livers will become increasingly important due to the obesity epidemic. As described by Jackson et al., drawing comparisons between outcomes of steatotic and non-steatotic livers is most often meaningless as the patient (and surgeon) do not get to decide simultaneous offers of each (81). Therefore the risk-benefit decision is really between survival with a steatotic transplanted liver, and no transplant at all. In a large US study that used national registry data, waitlisted recipients for whom a steatotic liver (≥30% macrosteatosis) offer was made but declined, 22.8% died on the waitlist and 17.6% were delisted. Overall, accepting a steatotic donor liver offer reduced the risk of mortality by 62% in comparison to declining it (81). Furthermore, the reduction in mortality seen with receiving a steatotic liver was greatest in the subgroup with the highest MELD score (81). Further demonstrating that steatotic livers offer a survival benefit in even the sickest of patients.
NMP has previously demonstrated benefits in the setting of steatotic livers, and probably represents one of the most frequent indications for its use (12, 13, 82). The experience with NMP preservation and transplantation of steatotic livers appears to be accruing, and the viability assessment provided by this modality clearly has a role in distinguishing those that will function adequately in the early post operative period. Our center’s experience is that one of the main reasons for a graft to be “orphaned” is steatosis, and only severely (>60% macrovesicular) steatotic livers fail the viability assessment. Minimizing the CIT of these grafts is paramount for a good outcome and NMP can facilitate this.
Prolonged Cold Ischemic Time
Reducing the cellular temperature to approximately 4°C slows cellular metabolism and therefore slows ATP consumption to approximately 10% of activity at normal body temperature (83). Nevertheless, ATP consumption continues and advanced donor age, BMI, and poor nutritional status may result in lower baseline ATP levels and therefore the organs tolerate the ischemic period poorly. The hypothermic conditions result in direct injury to cellular structures, such as the cytoskeleton and various organelles (84). Therefore the energy-conserving effect of cold preservation is partly offset by it non-physiological nature. An ischemia-reperfusion injury is the consequence of this preservation period and this impacts early graft function, and is the main reason for primary non-function (38). An improved understanding of the effect a cold ischemic period has on outcomes may have lead to the shortening of the cold ischemic period that is evident in registry data, and represents one of the main drivers for machine preservation technology (9).
Feng et al. demonstrated that in reference to a graft with an 8 h CIT, every additional hour resulted in a 1% decrease in 1 year graft survival (55). This suggests that the beneficial effect of minimizing CIT demonstrates a continuous pattern, rather than one with a clear threshold effect. Although NMP applied in a back-to-base model cannot alter the CIT associated with the donor procedure, it can abrogate the CIT that is associated with a lengthy recipient preparation period or hepatectomy. In our institution, we aim to keep the CIT at less than 6 h when applying NMP to suboptimal livers. This is achievable in the majority of instances, even if the graft is accepted very late in the retrieval process, as long as adequate personnel are available to prepare both the machine and the graft for perfusion. The timespan from arrival of the graft at our center until the commencement of NMP is approximately 2 h and this should be considered in the estimates of CIT.
Normothermic Machine Perfusion for High-Risk Graft-Recipient Combinations
In this section we discuss how we tie up the previously discussed issues together—the novel technology of NMP and the utility of this in the context of high-risk recipients who are significantly disadvantaged due to the scarcity of good-quality organs and timely transplantation of these candidates with marginal grafts. At our institution, Queen Elizabeth Hospital Birmingham, we have incorporated NMP technology into our service on a selected basis for the high-risk graft-recipient combination. The overall benefit of NMP technology over and above cold storage for standard graft-recipient combinations remains debatable. Although NMP is known to mitigate ischemia reperfusion injury and somewhat mitigate short-term beneficial outcomes, it is our belief that NMP should be utilized with a greater aim. Therefore, we utilize NMP in a manner that will allow high-risk recipients to benefit through improved graft access. The results of this approach have been previously published and presented at international conferences (14, 20). To update the current status of our program, between October 2018 and March 2022, we have perfused 107 liver grafts and 92 of these have proceeded to transplantation (Figure 4). Delivering a much needed organ transplantation service during the COVID-19 pandemic has provided additional challenges, however NMP has proven useful to overcome COVID testing requirements and hospital logistics regarding bed access. NMP provided a prolonged preservation time that would not have been possible, even in the standard graft-recipient combinations (85).
FIGURE 4
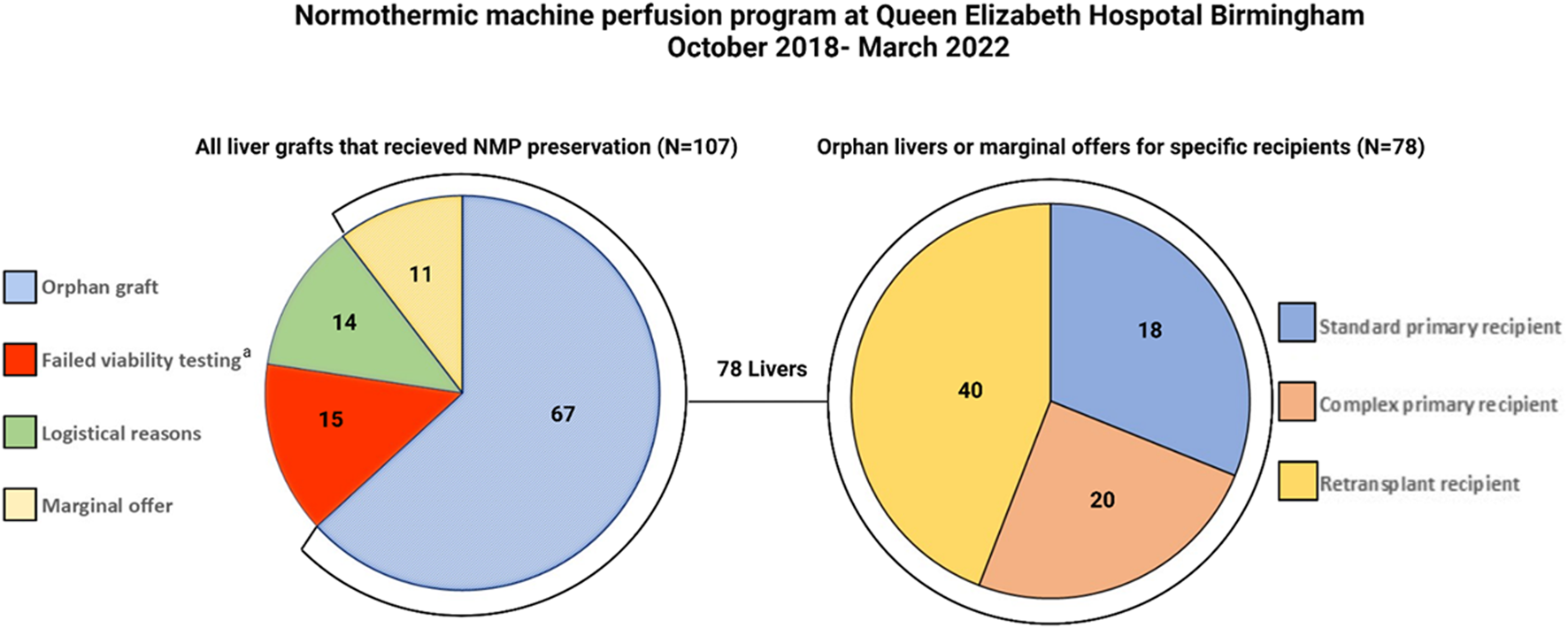
Pie charts demonstrating all grafts that underwent normothermic machine perfusion (NMP) at QEHB between October 2018 and April 2022 (left). The high-risk recipient-specific offers with marginal features (n = 11) or “orphan” grafts (n = 67) comprised 78/92 (85%) of the grafts transplanted. The right pie chart demonstrates that the majority of the livers went to either retransplant (40/78, 51%) or complex primary transplant recipients (18/78, 23%). Complex primary transplant recipients were those recipients with previous major hepatobiliary surgery or Yerdel grade ≥III portal vein thrombosis. aTwo grafts not transplanted due to recipient reasons and one due to equipment failure.
The ability to accept and safely transplant marginal and ‘orphan’ livers into high-risk recipients has been made possible with NMP due to a number of reasons. Firstly, the NMP viability assessment of a graft provides additional confidence that the graft will function adequately in the early post operative period. This is supported by the fact that PNF has not occurred in any of the 92 recipients of a liver preserved with NMP. Secondly, the ability to inspect and connect the liver to NMP expediently on arrival at our center allows the CIT to be kept to an acceptable minimum. This means that grafts that are declined at other transplant centers, in other parts of the UK, can still be reperfused within an adequate time period. The additional insult of a prolonged CIT on a graft with marginal attributes should not be underestimated, as undoubtedly there is an interaction between these variables as described previously. Finally, with the current national organ retrieval system in the UK being relatively inflexible about donor retrieval timing, it allows for a difficult operation to be performed during daylight hours with adequate and appropriate staffing.
In regards to graft physiology following NMP, we have experienced a lower than expected incidence of reperfusion syndrome and clinically significant early allograft dysfunction than would be expected given the graft and recipient characteristics (86). In the cohort of NMP-preserved grafts depicted in Figure 4, 40 out of the 78 marginal grafts were transplanted into patients undergoing retransplantation. The outcomes of the initial 26 patients in this cohort have been published previously (14). Since completing enrolment in this previous study and the reassuring results, we have performed an additional 14 retransplants using NMP-preserved grafts and all of these have reached at least 3 months follow-up. Therefore we have experience with a unique cohort of 40 patients undergoing retransplantation with NMP-preserved marginal liver grafts. The donor and graft characteristics of this group, in comparison to a retrospective cold storage control cohort transplanted at our institution over the last 5 years, are demonstrated in Table 1. Similar to our previously reported findings, the graft and patient survival did not differ between groups (Figure 5) despite the NMP group having significantly more steatotic grafts (moderate steatosis; 30% vs. 4%, P=<0.01), donors with peak ALT >1000 IU/L, and grafts declined by at least one other transplant center (78% vs. 26%, P=<0.01). Furthermore, in this expanded cohort, the peak alanine transaminase in the initial post operative 7 days was significantly lower (521 IU vs. 796 IU, p = 0.02) in the NMP group, and the EAD rate was not significantly different (47% vs. 37%). The rate of early acute rejection was higher (50% vs. 27%, p = 0.02) in the NMP cohort and has been discussed in a previous publication. We acknowledge that the incidence EAD rate is higher than previous reports, however this did not translate to early graft loss. Furthermore, a large majority were “biochemical” EAD due to raised bilirubin only (>177 mmol/L) in the presence of severe immune-mediated rejection, without any clinically relevant organ failure.
TABLE 1
| Donor | SCS group (N = 56) | NMP group (N = 40) | P |
|---|---|---|---|
| Donor age, (IQR) | 52 (44–69) | 50 (42–56) | 0.67 |
| Female | 25 (45%) | 27 (67%) | 0.03 |
| Donor BMI (IQR) | 24.9 (22.5–28.3) | 24.1 (21.4–27.7) | |
| Days in ICU (IQR) | 2 (2–4) | 3 (2–5) | 0.22 |
| DRI (IQR) | 1.55 (1.40–1.73) | 1.57 (1.38–1.69) | 0.92 |
| DLI (IQR) | 1.05 (0.92–1.21) | 0.99 (0.86–1.13) | 0.16 |
| Inotrope requirement | 48 (86%) | 36 (90%) | 0.53 |
| Smoker | |||
| History of alcohol excess | 11 (20%) | 15 (38%) | 0.07 |
| Donor cardiac arrest | 24 (44%) | 13 (32%) | 0.27 |
| Downtime minutes (IQR) | 30 (8–48) | 38 (28–52) | 0.142 |
| Liver biochemistry | |||
| Peak ALT, IU/L (IQR) | 53 (21–99) | 109 (40–669) | <0.01 |
| Peak bilirubin, mg/dL (IQR) | 9 (7–16) | 13 (8–20) | 0.03 |
| Donor ALT ≥1000 IU/L | 0 (0%) | 9 (23%) | <0.01 |
| Graft | SCS group (N = 56) | NMP group (N = 40) | P |
| Declined by at least 1 other centera | 14 (26%) | 31 (78%) | <0.01 |
| Steatosis | <0.01 | ||
| None | 40 (73%) | 21 (53%) | |
| Mild | 13 (24%) | 7 (18%) | |
| Moderate | 2 (4%) | 12 (30%) | |
| Cold ischemic time, min (IQR) | 482 (409–596) | 372 (325–425) | <0.01 |
| Perfusion time, min (IQR) | — | 759 (488–953) | N/A |
| Total preservationb, min (IQR) | 482 (409–596) | 1107 (746–1330) | <0.01 |
| Recipient | SCS group (N = 56) | NMP group (N = 40) | P |
| Age (IQR) | 43 (29–56) | 36 (24–50) | 0.05 |
| UKELD | 58 (55–63) | 58 (53–61) | 0.73 |
| MELD | 19 (14–25) | 21 (13–26) | 0.82 |
| Number of previous grafts | 0.06 | ||
| One (first retransplant) | 49 (87%) | 29 (72%) | |
| Two (second retransplant | 7 (13%) | 9 (21%) | |
| Three (third retransplant) | 0 (0%) | 2 (7%) | |
| Indication | 0.40 | ||
| Hepatic artery thrombosis | 17 (30%) | 14 (35%) | |
| Chronic rejection | 5 (9%) | 8 (20%) | |
| Biliary complications | 18 (32%) | 9 (22%) | |
| Disease recurrence | 13 (23%) | 6 (15%) | |
| Waitlist duration (days) | 72 (26–151) | 235 (60–423) | <0.01 |
| Follow up (Median, months) | 40 (25–56) | 21 (11–29) | <0.01 |
Donor, graft and recipient characteristics.
Categorical variables compared with Chi-square test. Independent sample T-test used to compare continues variables that were normally distributed. Mann -Whitney U test used to compare normally distributed continuous variables. AL, alanine aminotransferase; BMI, body mass index; ICU, intensive care unit; DRI, donor risk index; DLI, donor liver index; UKELD, United Kingdom model for end stage liver disease; MELD, Model for end stage liver disease; SCS, Static cold storage; NMP, Normothermic machine Perfusion.
Reason related to donor or graft quality.
Total preservation time comprised cold ischemic time and perfusion time.
FIGURE 5
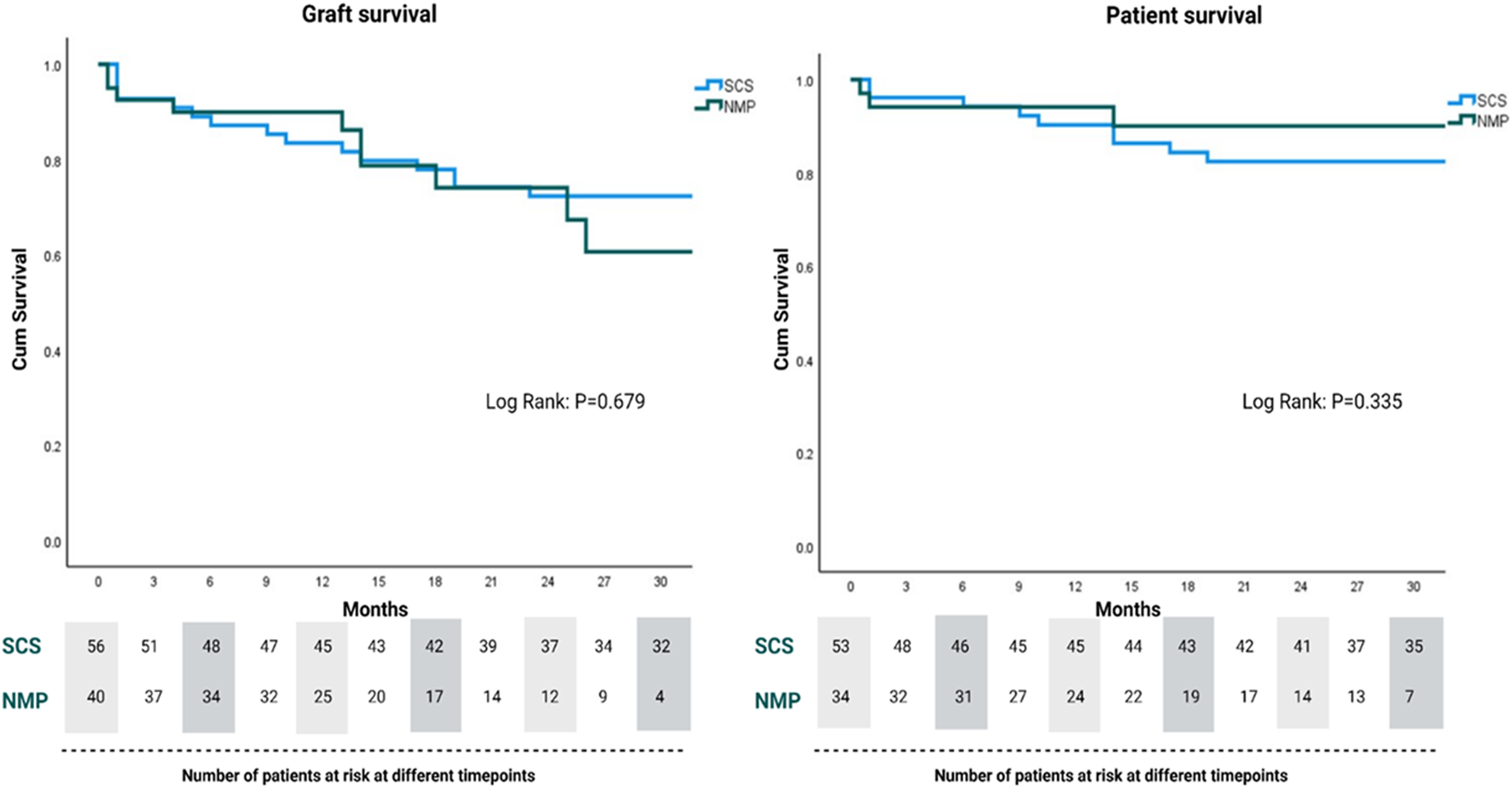
Graft and patient survival for normothermic machine perfusion (NMP) and static cold storage (SCS)-preserved grafts for retransplant recipients.
Conclusion
The transplantability of a liver graft has long been a subjective assessment. It is understandable that surgeons may therefore err on the side of caution, in an effort to do no harm. This is of course particularly important when the predicted surgical risks are already high. The ability of NMP technology to extend liver graft preservation time can allow more complex recipients to be transplanted in a controlled and safe manner. Furthermore, it can safely facilitate the usage of liver grafts for these recipients that would otherwise not have been considered. Evidence supporting the use of NMP for marginal organs and high-risk recipients is starting to emerge with promising data in the retransplantation setting. The problem inherent with researching this topic is the lack of appropriate controls for comparison. Retrospective control cohorts, or even propensity matching, have their limitations as under different preservation conditions these grafts would not have been transplanted previously (87). The benefits of NMP in the setting of a suboptimal graft and high-risk recipient justify the additional resources required for this technology. Similar to the improved understanding of cold preservation techniques throughout the second half of the 20th century, the application of NMP continues to be refined through research at many centers around the world. As experience with machine perfusion technology grows, this will hopefully translate into improved transplant outcomes.
Data Sharing Agreement
Data collected for this study, including individual participant data and the data dictionary, will be made available to others at publication. The data will be in an anonymized form to protect participants’ privacy. The authorship agrees to provide access to all additional study documents.
Statements
Author contributions
AH wrote and edited the first draft of the manuscript. AN, GC, IP, DS, and YO reviewed and edited the manuscript. MP and HH conceptualized, reviewed, and edited the manuscript.
Funding
The funding provided for normothermic machine perfusion consumables generously donated by the Ann Fox Foundation, under the umbrella of University Hospital Birmingham Charities.
Acknowledgments
Figures 1–5 were created with biorender.com. AH would like to acknowledge the funding received in the form of the Catherine Marie Enright research scholarship from the Royal Australasian College of Surgeons. YO would like to acknowledge the funding provided by the Sir Jules Thorn Biomedical Research Charity for his program of research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CIT, cold ischemic time; DBD, deceased brain death; DCD, deceased circulatory death; DLI, donor liver index; DRI, donor risk index; GGT, gamma-glutamyl transferase; HBI, hypoxic brain injury; HCV, hepatitis C virus; ICU, intensive care unit; ITBL, ischemic-type biliary lesions; MELD, model of end stage liver disease; NMP, normothermic machine perfusion; PNF, primary non-function; SCS, static cold storage; UK, United Kingdom; UKELD, United Kingdom model of end stage liver disease; US, United States.
References
1.
Starzl TE Marchioro TL Vonkaulla KN Hermann G Brittain RS Waddell WR . Homotransplantation of the Liver in Humans. Surg Gynecol Obstet (1963). 117:659–76.
2.
Calne RY Williams R . Liver Transplantation in Man-I, Observations on Technique and Organization in Five Cases. BMJ (1968). 4(5630):535–40. 10.1136/bmj.4.5630.535
3.
AuthorAnonymous . A Definition of Irreversible Coma. Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA (1968). 205(6):337–40.
4.
Hibi T Rela M Eason JD Line P-D Fung J Sakamoto S et al Liver Transplantation for Colorectal and Neuroendocrine Liver Metastases and Hepatoblastoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation (2020). 104(6):1131–5. 10.1097/tp.0000000000003118
5.
Panayotova G Lunsford KE Latt NL Paterno F Guarrera JV Pyrsopoulos N . Expanding Indications for Liver Transplantation in the Era of Liver Transplant Oncology. World J Gastrointest Surg (2021). 13(5):392–405. 10.4240/wjgs.v13.i5.392
6.
Vimalesvaran S Dhawan A . Liver Transplantation for Pediatric Inherited Metabolic Liver Diseases. World J Hepatol (2021). 13(10):1351–66. 10.4254/wjh.v13.i10.1351
7.
Dueland S Syversveen T Solheim JM Solberg S Grut H Bjørnbeth BA et al Survival Following Liver Transplantation for Patients with Nonresectable Liver-Only Colorectal Metastases. Ann Surg (2020). 271(2):212–8. 10.1097/sla.0000000000003404
8.
Cambridge WA Fairfield C Powell JJ Harrison EM Søreide K Wigmore SJ et al Meta-analysis and Meta-Regression of Survival after Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Ann Surg (2021). 273(2):240–50. 10.1097/sla.0000000000003801
9.
Kwong A Kim WR Lake JR Smith JM Schladt DP Skeans MA et al OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transpl (2020). 20(Suppl. s1):193–299. 10.1111/ajt.15674
10.
Silveira F Silveira FP Freitas ACT Coelho JCU Ramos EJB Macri MM et al Liver Transplantation: Survival and Indexes of Donor-Recipient Matching. Rev Assoc Med Bras (1992) (2021). 67(5):690–5. 10.1590/1806-9282.20201088
11.
Lozanovski VJ Probst P Arefidoust A Ramouz A Aminizadeh E Nikdad M et al Prognostic Role of the Donor Risk Index, the Eurotransplant Donor Risk Index, and the Balance of Risk Score on Graft Loss after Liver Transplantation. Transpl Int (2021). 34(5):778–800. 10.1111/tri.13861
12.
Cardini B Oberhuber R Fodor M Hautz T Margreiter C Resch T et al Clinical Implementation of Prolonged Liver Preservation and Monitoring through Normothermic Machine Perfusion in Liver Transplantation. Transplantation (2020). 104(9):1917–28. 10.1097/tp.0000000000003296
13.
Mergental H Laing RW Kirkham AJ Perera MTPR Boteon YL Attard J et al Transplantation of Discarded Livers Following Viability Testing with Normothermic Machine Perfusion. Nat Commun (2020). 11(1):2939. 10.1038/s41467-020-16251-3
14.
Hann A Lembach H Nutu A Dassanayake B Tillakaratne S McKay SC et al Outcomes of Normothermic Machine Perfusion of Liver Grafts in Repeat Liver Transplantation (NAPLES Initiative). Br J Surg (2022). 109:372. 10.1093/bjs/znab475
15.
van Rijn R Schurink IJ de Vries Y van den Berg AP Cortes Cerisuelo M Darwish Murad S et al Hypothermic Machine Perfusion in Liver Transplantation - A Randomized Trial. N Engl J Med (2021). 384(15):1391–401. 10.1056/nejmoa2031532
16.
Hessheimer AJ de la Rosa G Gastaca M Ruíz P Otero A Gómez M et al Abdominal Normothermic Regional Perfusion in Controlled Donation after Circulatory Determination of Death Liver Transplantation: Outcomes and Risk Factors for Graft Loss. Am J Transpl (2021). 22:1169. 10.1111/ajt.16899
17.
Dutkowski P Oberkofler CE Slankamenac K Puhan MA Schadde E Müllhaupt B et al Are There Better Guidelines for Allocation in Liver Transplantation? A Novel Score Targeting justice and Utility in the Model for End-Stage Liver Disease Era. Ann Surg (2011). 254(5):745–54. 10.1097/sla.0b013e3182365081
18.
Ritschl PV Wiering L Dziodzio T Jara M Kruppa J Schoeneberg U et al The Effects of MELD-Based Liver Allocation on Patient Survival and Waiting List Mortality in a Country with a Low Donation Rate. J Clin Med (2020). 9(6):1929. 10.3390/jcm9061929
19.
Tzakis A Todo S Starzl TE . Orthotopic Liver Transplantation with Preservation of the Inferior Vena Cava. Ann Surg (1989). 210(5):649–52. 10.1097/00000658-198911000-00013
20.
Lembach H McKay S Hann A Carvalheiro A Boteon Y Alzoubi M et al Naples Study (Normothermic Machine Perfusion of the Liver to Enable the Sickest First): Preliminary Results. Transplantation (2020). 104:S248. 10.1097/01.tp.0000699716.34046.6d
21.
Buchholz BM Gerlach UA Chandrabalan VV Hodson J Gunson BK Mergental H et al Revascularization Time in Liver Transplantation: Independent Prediction of Inferior Short- and Long-Term Outcomes by Prolonged Graft Implantation. Transplantation (2018). 102(12):2038–55. 10.1097/tp.0000000000002263
22.
Al-Kurd A Kitajima T Delvecchio K Tayseer Shamaa M Ivanics T Yeddula S et al Short Recipient Warm Ischemia Time Improves Outcomes in Deceased Donor Liver Transplantation. Transpl Int (2021). 34(8):1422–32. 10.1111/tri.13962
23.
Vivarelli M Benedetti Cacciaguerra A Lerut J Lanari J Conte G Pravisani R et al Infrarenal versus Supraceliac Aorto-Hepatic Arterial Revascularisation in Adult Liver Transplantation: Multicentre Retrospective Study. Updates Surg (2020). 72(3):659–69. 10.1007/s13304-020-00839-x
24.
Buchholz BM Khan S David MD Gunson BK Isaac JR Roberts KJ et al Retransplantation in Late Hepatic Artery Thrombosis: Graft Access and Transplant Outcome. Transplant Direct (2017). 3(8):e186. 10.1097/txd.0000000000000705
25.
Hung K Gralla J Dodge JL Bambha KM Dirchwolf M Rosen HR et al Optimizing Repeat Liver Transplant Graft Utility through Strategic Matching of Donor and Recipient Characteristics. Liver Transpl (2015). 21(11):1365–73. 10.1002/lt.24138
26.
Croome KP Marotta P Wall WJ Dale C Levstik MA Chandok N et al Should a Lower Quality Organ Go to the Least Sick Patient? Model for End-Stage Liver Disease Score and Donor Risk index as Predictors of Early Allograft Dysfunction. Transplant Proc (2012). 44(5):1303–6. 10.1016/j.transproceed.2012.01.115
27.
Reiling J Butler N Simpson A Hodgkinson P Campbell C Lockwood D et al Assessment and Transplantation of Orphan Donor Livers: A Back‐to‐Base Approach to Normothermic Machine Perfusion. Liver Transpl (2020). 26(12):1618–28. 10.1002/lt.25850
28.
Hann A Lembach H Nutu A Mergental H Isaac JL Isaac JR et al Assessment of Deceased Brain Dead Donor Liver Grafts via Normothermic Machine Perfusion: Lactate Clearance Time Threshold Can Be Safely Extended to 6 hours. Liver Transpl (2021). 28:493. 10.1002/lt.26317
29.
Hann A Lembach H Alzoubi M McKay SC Hartog H Neil DAH et al Hepatocyte Necrosis on Liver Allograft Biopsy: Normothermic Machine Perfusion is the Ideal Platform for Using These Grafts in High-Risk Recipients. Clin Transpl (2021). 35(7):e14380. 10.1111/ctr.14380
30.
Carvalheiro AP McKay SC Bartlett DC Dronavalli VB Thilekertane S Dassanayake B et al Novel Use of Normothermic Machine Perfusion of the Liver: A Strategy to Mitigate Unexpected Clinical Events. Transplantation (2020). 104(9):e281–e282. 10.1097/tp.0000000000003288
31.
Jayant K Reccia I Shapiro AMJ . Normothermic Ex-Vivo Liver Perfusion: where Do We Stand and where to Reach?Expert Rev Gastroenterol Hepatol (2018). 12(10):1045–58. 10.1080/17474124.2018.1505499
32.
Matton APM de Vries Y Burlage LC van Rijn R Fujiyoshi M de Meijer VE et al Biliary Bicarbonate, pH, and Glucose are Suitable Biomarkers of Biliary Viability during Ex Situ Normothermic Machine Perfusion of Human Donor Livers. Transplantation (2019). 103(7):1405–13. 10.1097/tp.0000000000002500
33.
Watson CJE Kosmoliaptsis V Randle LV Gimson AE Brais R Klinck JR et al Normothermic Perfusion in the Assessment and Preservation of Declined Livers before Transplantation: Hyperoxia and Vasoplegia-Important Lessons from the First 12 Cases. Transplantation (2017). 101(5):1084–98. 10.1097/tp.0000000000001661
34.
Watson CJE Kosmoliaptsis V Pley C Randle L Fear C Crick K et al Observations on the Ex Situ Perfusion of Livers for Transplantation. Am J Transpl (2018). 18(8):2005–20. 10.1111/ajt.14687
35.
Watson CJE Jochmans I . From "Gut Feeling" to Objectivity: Machine Preservation of the Liver as a Tool to Assess Organ Viability. Curr Transpl Rep (2018). 5(1):72–81. 10.1007/s40472-018-0178-9
36.
Serifis N Matheson R Cloonan D Rickert CG Markmann JF Coe TM . Machine Perfusion of the Liver: A Review of Clinical Trials. Front Surg (2021). 8:625394. 10.3389/fsurg.2021.625394
37.
Mergental H Laing RW Hodson J Boteon YL Attard JA Walace LL et al Introduction of the Concept of Diagnostic Sensitivity and Specificity of Normothermic Perfusion Protocols to Assess High-Risk Donor Livers. Liver Transpl (2021). 28:794. 10.1002/lt.26326
38.
Hartog H Hann A Perera MTPR . Primary Nonfunction of the Liver Allograft. Transplantation (2022). 106(1):117–28. 10.1097/tp.0000000000003682
39.
Markmann JF Abouljoud MS Ghobrial RM Bhati CS Pelletier SJ Lu AD et al Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg (2022). 157:189. 10.1001/jamasurg.2021.6781
40.
Fodor M Cardini B Peter W Weissenbacher A Oberhuber R Hautz T et al Static Cold Storage Compared with Normothermic Machine Perfusion of the Liver and Effect on Ischaemic-type Biliary Lesions after Transplantation: A Propensity Score-Matched Study. Br J Surg (2021). 108(9):1082–9. 10.1093/bjs/znab118
41.
Cohen B Matot I . Aged Erythrocytes: A fine Wine or Sour Grapes?Br J Anaesth (2013). 111(Suppl. 1):i62–i70. 10.1093/bja/aet405
42.
Passarella S Schurr A . l-Lactate Transport and Metabolism in Mitochondria of Hep G2 Cells-The Cori Cycle Revisited. Front Oncol (2018). 8:120. 10.3389/fonc.2018.00120
43.
Mergental H Perera MTPR Laing RW Muiesan P Isaac JR Smith A et al Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am J Transpl (2016). 16(11):3235–45. 10.1111/ajt.13875
44.
NHS Blood and Transplant. Annual Report on the National Organ Retrieval Service (NORS). Reort for 2020/2021. UK: NHS Blood and Transplant (2021).
45.
Marcon F Schlegel A Bartlett DC Kalisvaart M Bishop D Mergental H et al Utilization of Declined Liver Grafts Yields Comparable Transplant Outcomes and Previous Decline Should Not Be a Deterrent to Graft Use. Transplantation (2018). 102(5):e211–e218. 10.1097/tp.0000000000002127
46.
McCormack L Quiñonez E Ríos MM Capitanich P Goldaracena N Cabo JK et al Rescue Policy for Discarded Liver Grafts: A Single-centre Experience of Transplanting Livers 'that Nobody Wants'. HPB (2010). 12(8):523–30. 10.1111/j.1477-2574.2010.00193.x
47.
NHS Blood and Transplant. Transplantation of Organs from Deceased Donors with Cancer or a History of Cancer. Bristol, United Kingdom: Advisory Comittee on the Safety of Blood, Tissues and Organs-SaBTO (2020). Contract No.: 2.1.
48.
NHS Blood and Transplant. Microbiological Safety Guidelines. Bristol, United Kingdom: Advisory Comittee on the Safety of Blood, Tissues and Organs (2020).
49.
Escartín A Castro E Dopazo C Bueno J Bilbao I Margarit C . Analysis of Discarded Livers for Transplantation. Transpl Proc (2005). 37(9):3859–60. 10.1016/j.transproceed.2005.08.050
50.
Mangus RS Fridell JA Kubal CA Davis JP Joseph Tector A . Elevated Alanine Aminotransferase (ALT) in the Deceased Donor: Impact on Early post-transplant Liver Allograft Function. Liver Int (2015). 35(2):524–31. 10.1111/liv.12508
51.
Petersen KF Befroy D Dufour S Dziura J Ariyan C Rothman DL et al Mitochondrial Dysfunction in the Elderly: Possible Role in Insulin Resistance. Science (2003). 300(5622):1140–2. 10.1126/science.1082889
52.
Slawik M Vidal-Puig AJ . Lipotoxicity, Overnutrition and Energy Metabolism in Aging. Ageing Res Rev (2006). 5(2):144–64. 10.1016/j.arr.2006.03.004
53.
Mahrouf-Yorgov M de l'Hortet AC Cosson C Slama A Abdoun E Guidotti J-E et al Increased Susceptibility to Liver Fibrosis with Age is Correlated with an Altered Inflammatory Response. Rejuvenation Res (2011). 14(4):353–63. 10.1089/rej.2010.1146
54.
Giorgakis E Khorsandi SE Mathur AK Burdine L Jassem W Heaton N . Comparable Graft Survival Is Achievable with the Usage of Donation after Circulatory Death Liver Grafts from Donors at or above 70 Years of Age: A Long‐term UK National Analysis. Am J Transpl (2021). 21(6):2200–10. 10.1111/ajt.16409
55.
Feng S Goodrich NP Bragg-Gresham JL Dykstra DM Punch JD DebRoy MA et al Characteristics Associated with Liver Graft Failure: The Concept of a Donor Risk index. Am J Transpl (2006). 6(4):783–90. 10.1111/j.1600-6143.2006.01242.x
56.
Gao Q Mulvihill MS Scheuermann U Davis RP Yerxa J Yerokun BA et al Improvement in Liver Transplant Outcomes from Older Donors: A US National Analysis. Ann Surg (2019). 270(2):333–9. 10.1097/sla.0000000000002876
57.
Ghinolfi D Marti J De Simone P Lai Q Pezzati D Coletti L et al Use of Octogenarian Donors for Liver Transplantation: A Survival Analysis. Am J Transpl (2014). 14(9):2062–71. 10.1111/ajt.12843
58.
Singal AK Bataller R Ahn J Kamath PS Shah VH . ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol (2018). 113(2):175–94. 10.1038/ajg.2017.469
59.
Durand F Renz JF Alkofer B Burra P Clavien P-A Porte RJ et al Report of the Paris Consensus Meeting on Expanded Criteria Donors in Liver Transplantation. Liver Transpl (2008). 14(12):1694–707. 10.1002/lt.21668
60.
Mangus RS Kubal CA Fridell JA Pena JM Frost EM Joseph Tector A . Alcohol Abuse in Deceased Liver Donors: Impact on post-transplant Outcomes. Liver Int (2015). 35(1):171–5. 10.1111/liv.12484
61.
Silberhumer GR Rahmel A Karam V Gonen M Gyoeri G Kern B et al The Difficulty in Defining Extended Donor Criteria for Liver Grafts: The Eurotransplant Experience. Transpl Int (2013). 26(10):990–8. 10.1111/tri.12156
62.
Eurotransplant. Organ Match Characteristics. Available from: https://www.eurotransplant.org/organs/liver/ (Accessed February 1, 2020).
63.
Dufour DR Lott JA Nolte FS Gretch DR Koff RS Seeff LB . Diagnosis and Monitoring of Hepatic Injury. I. Performance Characteristics of Laboratory Tests. Clin Chem (2000). 46(12):2027–49. 10.1093/clinchem/46.12.2027
64.
Strnad P Tacke F Koch A Trautwein C . Liver - Guardian, Modifier and Target of Sepsis. Nat Rev Gastroenterol Hepatol (2017). 14(1):55–66. 10.1038/nrgastro.2016.168
65.
Martins PN Rawson A Movahedi B Brüggenwirth IMA Dolgin NH Martins AB et al Single-Center Experience with Liver Transplant Using Donors with Very High Transaminase Levels. Exp Clin Transpl (2019). 17(4):498–506. 10.6002/ect.2017.0172
66.
Collett D Friend PJ Watson CJE . Factors Associated with Short- and Long-Term Liver Graft Survival in the United Kingdom: Development of a UK Donor Liver Index. Transplantation (2017). 101(4):786–92. 10.1097/tp.0000000000001576
67.
Misar A McLin VA Calinescu AM Wildhaber BE . Impact of Length of Donor ICU Stay on Outcome of Patients after Pediatric Liver Transplantation with Whole and Ex Situ Split Liver Grafts. Pediatr Transpl (2021). 26:e14186. 10.1111/petr.14186
68.
Cerutti E Stratta C Romagnoli R Serra R Lepore M Fop F et al Bacterial- and Fungal-Positive Cultures in Organ Donors: Clinical Impact in Liver Transplantation. Liver Transpl (2006). 12(8):1253–9. 10.1002/lt.20811
69.
Strasberg SM Howard TK Molmenti EP Hertl M . Selecting the Donor Liver: Risk Factors for Poor Function after Orthotopic Liver Transplantation. Hepatology (1994). 20(4 Pt 1):829–38. 10.1002/hep.1840200410
70.
Geenen ILA Sosef MN Shun A Crawford M Gallagher J Strasser S et al Usage and Outcomes of Deceased Donor Liver Allografts with Preprocurement Injury from blunt Trauma. Liver Transpl (2009). 15(3):321–5. 10.1002/lt.21687
71.
Croome KP Lee DD Taner CB . The "Skinny" on Assessment and Utilization of Steatotic Liver Grafts: A Systematic Review. Liver Transpl (2019). 25(3):488–99. 10.1002/lt.25408
72.
Steggerda JA Kim IK Malinoski D Klein AS Bloom MB . Regional Variation in Utilization and Outcomes of Liver Allografts from Donors with High Body Mass Index and Graft Macrosteatosis: A Role for Liver Biopsy. Transplantation (2019). 103(1):122–30. 10.1097/tp.0000000000002379
73.
Fishbein TM Fiel MI Emre S Cubukcu O Guy SR Schwartz ME et al Use of Livers with Microvesicular Fat Safely Expands the Donor Pool. Transplantation (1997). 64(2):248–51. 10.1097/00007890-199707270-00012
74.
Andert A Ulmer TF Schöning W Kroy D Hein M Alizai PH et al Grade of Donor Liver Microvesicular Steatosis Does Not Affect the Postoperative Outcome after Liver Transplantation. Hepatobiliary Pancreat Dis Int (2017). 16(6):617–23. 10.1016/s1499-3872(17)60064-x
75.
de Graaf EL Kench J Dilworth P Shackel NA Strasser SI Joseph D et al Grade of Deceased Donor Liver Macrovesicular Steatosis Impacts Graft and Recipient Outcomes More Than the Donor Risk Index. J Gastroenterol Hepatol (2012). 27(3):540–6. 10.1111/j.1440-1746.2011.06844.x
76.
Spitzer AL Lao OB Dick AAS Bakthavatsalam R Halldorson JB Yeh MM et al The Biopsied Donor Liver: Incorporating Macrosteatosis into High-Risk Donor Assessment. Liver Transpl (2010). 16(7):874–84. 10.1002/lt.22085
77.
Kulik U Lehner F Klempnauer J Borlak J . Primary Non-function Is Frequently Associated with Fatty Liver Allografts and High Mortality after Re-transplantation. Liver Int (2017). 37(8):1219–28. 10.1111/liv.13404
78.
Yersiz H Lee C Kaldas FM Hong JC Rana A Schnickel GT et al Assessment of Hepatic Steatosis by Transplant Surgeon and Expert Pathologist: A Prospective, Double-Blind Evaluation of 201 Donor Livers. Liver Transpl (2013). 19(4):437–49. 10.1002/lt.23615
79.
McCormack L Dutkowski P El-Badry AM Clavien P-A . Liver Transplantation Using Fatty Livers: Always Feasible?J Hepatol (2011). 54(5):1055–62. 10.1016/j.jhep.2010.11.004
80.
Chu MJJ Dare AJ Phillips ARJ Bartlett ASJR . Donor Hepatic Steatosis and Outcome after Liver Transplantation: A Systematic Review. J Gastrointest Surg (2015). 19(9):1713–24. 10.1007/s11605-015-2832-1
81.
Jackson KR Bowring MG Holscher C Haugen CE Long JJ Liyanage L et al Outcomes after Declining a Steatotic Donor Liver for Liver Transplant Candidates in the United States. Transplantation (2020). 104(8):1612–8. 10.1097/tp.0000000000003062
82.
Lai Q Ruberto F Pawlik TM Pugliese F Rossi M . Use of Machine Perfusion in Livers Showing Steatosis Prior to Transplantation: a Systematic Review. Updates Surg (2020). 72(3):595–604. 10.1007/s13304-020-00797-4
83.
Bellini MI Yiu J Nozdrin M Papalois V . The Effect of Preservation Temperature on Liver, Kidney, and Pancreas Tissue ATP in Animal and Preclinical Human Models. J Clin Med (2019). 8(9):1421. 10.3390/jcm8091421
84.
Fondevila C Busuttil RW Kupiec-Weglinski JW . Hepatic Ischemia/reperfusion Injury-A Fresh Look. Exp Mol Pathol (2003). 74(2):86–93. 10.1016/s0014-4800(03)00008-x
85.
Lembach H Hann A McKay SC Hartog H Vasanth S El-Dalil P et al Resuming Liver Transplantation amid the COVID-19 Pandemic. Lancet Gastroenterol Hepatol (2020). 5(8):725–6. 10.1016/s2468-1253(20)30187-4
86.
Hann A Lembach H Carvalheiro A Boteon Y McKay S Kadam P et al Normothermic Machine Perfusion of Marginal Liver Allografts is Associated with A Low Incidence of Post Reperfusion Syndrome in High Risk Recipients. Transplantation (2020). 104:S60. 10.1097/01.tp.0000698552.38741.a1
87.
Hann AA Raza D Sneiders H Hartog H Perera MTP Mirza DF et al Comment on: Static Cold Storage Compared with Normothermic Machine Perfusion of the Liver and Effect on Ischaemic-type Biliary Lesions after Transplantation: A Propensity-Score Matched Study. Br J Surg (2021). 109(1):e12–e13. 10.1093/bjs/znab349
Summary
Keywords
transplant, normothermic machine perfusion, liver, preservation, marginal, retransplant
Citation
Hann A, Nutu A, Clarke G, Patel I, Sneiders D, Oo YH, Hartog H and Perera MTPR (2022) Normothermic Machine Perfusion—Improving the Supply of Transplantable Livers for High-Risk Recipients. Transpl Int 35:10460. doi: 10.3389/ti.2022.10460
Received
23 February 2022
Accepted
04 May 2022
Published
31 May 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Hann, Nutu, Clarke, Patel, Sneiders, Oo, Hartog and Perera.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Thamara P. R. Perera, thamara.perera@uhb.nhs.uk
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.