Abstract
Since candidates with comorbidities are increasingly referred for lung transplantation, knowledge about comorbidities and their cumulative effect on outcomes is scarce. We retrospectively collected pretransplant comorbidities of all 513 adult recipients transplanted at our center between 1992–2019. Multiple logistic- and Cox regression models, adjusted for donor-, pre- and peri-operative variables, were used to detect independent risk factors for primary graft dysfunction grade-3 at 72 h (PGD3-T72), onset of chronic allograft dysfunction grade-3 (CLAD-3) and survival. An increasing comorbidity burden measured by Charleston-Deyo-Index was a multivariable risk for survival and PGD3-T72, but not for CLAD-3. Among comorbidities, congestive right heart failure or a mean pulmonary artery pressure >25 mmHg were independent risk factors for PGD3-T72 and survival, and a borderline risk for CLAD-3. Left heart failure, chronic atrial fibrillation, arterial hypertension, moderate liver disease, peptic ulcer disease, gastroesophageal reflux, diabetes with end organ damage, moderate to severe renal disease, osteoporosis, and diverticulosis were also independent risk factors for survival. For PGD3-T72, a BMI>30 kg/m2 was an additional independent risk. Epilepsy and a smoking history of the recipient of >20packyears are additional independent risk factors for CLAD-3. The comorbidity profile should therefore be closely considered for further clinical decision making in candidate selection.
Introduction
Comorbidities in lung transplant candidates have increasingly been accepted over the last decades in parallel with steadily increasing numbers of lung transplantation procedures over time. This broadening of acceptable candidates was partly supported by the International Society for Heart and Lung Transplantation (ISHLT) consensus report for the selection of lung transplant candidates, published in 1998 and updated in 2006, 2014 (1) and 2021 (2). However, these consensus reports are based mainly on expert opinion. Strong evidence about comorbidities and their impact on primary graft dysfunction (PGD), chronic allograft dysfunction (CLAD), and survival are still missing. Moreover, almost nothing is known about the cumulative effect of comorbidities in a potential lung transplant candidate. In other fields of medicine, the cumulative effect of comorbidities for prognostic assessment has been extensively studied. One of the most commonly used comorbidity models is the Charlson-Comorbidity-Index introduced in 1987 (3). This index is based on comorbid conditions with varying assigned weights, resulting in a composite score. As increasing age was shown to be more an expression of accumulation of comorbidities than an actual risk factor per se, an age independent version, the Charlson-Deyo-Index (CDI)(4) was proposed. Among transplant patients, the CDI and its derivates has shown to be predictive in recipients undergoing renal transplantation (5, 6) and liver transplantation (7, 8).
In the era of organ shortage, it is of paramount importance to know which patient and at which time point will benefit from lung transplantation for an extended time period. We investigated the impact of a large variety of pretransplant comorbidities among our recipients transplanted at our center in respect to PGD, CLAD and survival. For cumulative comorbidity conditions, we additionally evaluated the CDI for the same outcomes.
Methods
We systematically, retrospectively collected data from medical records of all adult recipients and their corresponding donors transplanted at the University Hospital of Zurich between 11/1992 and 12/2019, with last follow-up in 01/2022. Recipient selection was based on a liberal use of the updated ISHLT consensus document (1). All comorbidity variables were based on the most immediate pretransplantation data. Follow-up of the recipients was performed in our outpatient department or in close quarterly to half-yearly exchange with other institutions.
Definition of the Charlson-Deyo-Index
This index (4) is age independent and estimates the impact of multiple comorbidities. It considers 19 comorbid conditions (ranging from 1 to 6 points), of which 1 point was always reserved by the chronic pulmonary disease in each of our recipients. All included comorbidities and their assigned points are listed in Tables 1–3. An increasing score of points represents an increasing category of risk.
TABLE 1
| N = 513 | Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | Model | HR | 95% CI | p | ||
| Recipient Characteristics | ||||||||
| Age (median, range) | 49 (18–70) | 1.02 | 1.02–1.03 | 0.000 | A, B, C, D | 1.01 | 1.00–1.02 | 0.004 |
| Sex male | 270 (52.6%) | 1.14 | 0.92–1.41 | 0.220 | ||||
| Diagnosis | ||||||||
| Cystic fibrosis | 156 (30.4%) | 0.57 | 0.45–0.73 | 0.000 | ||||
| Idiopathic pulmonary arterial hypertension | 27 (5.2%) | 1.33 | 0.86–2.07 | 0.205 | ||||
| Emphysema | 155 (30.2%) | 1.19 | 0.95–1.49 | 0.133 | ||||
| Idiopathic pulmonary fibrosis | 111 (21.6%) | 1.54 | 1.21–1.97 | 0.001 | ||||
| Other | 64 (12.5%) | |||||||
| Smoking (pack years) (median, range) | 0 (0–120) | 1.01 | 1.00–1.01 | 0.006 | ||||
| >20py | 187 (36.5%) | 1.34 | 1.08–1.66 | 0.009 | ||||
| Waitlist (days) (median, range) | 150 (0–1965) | 1.00 | 1.00–1.00 | 0.525 | ||||
| Recipient Comorbidities | ||||||||
| Any coronary artery disease | 58 (11.3%) | 1.71 | 1.23–2.37 | 0.001 | ||||
| Myocardial infarctiona(1pt) | 7 (1.4%) | 2.44 | 1.01–5.93 | 0.048 | ||||
| Postinterventional coronary disease (stent) | 16 (3.1) | 1.47 | 0.82–2.61 | 0.194 | ||||
| Coronary disease mild | 43 (8.4%) | 1.68 | 1.16–2.44 | 0.006 | ||||
| Congestive heart failurea(1pt) | 267 (52.0%) | 2.13 | 1.71–2.64 | 0.000 | A | 1.91 | 1.53–2.40 | 0.004 |
| Right heart failure | 262 (51.1%) | 2.04 | 1.65–2.53 | 0.000 | C | 1.81 | 1.45–2.28 | 0.000 |
| mPAP (median, range) | 28 (17–82) | 1.02 | 1.01–1.03 | 0.000 | B, C | 1.64 | 1.31–2.06 | 0.000 |
| >25 mmHg | 264 (51.5%) | 1.91 | 1.54–2.37 | 0.000 | ||||
| Left heart failure | 12 (2.3%) | 3.62 | 1.97–6.64 | 0.000 | C | 2.07 | 1.11–3.87 | 0.023 |
| Chronic atrial fibrillation | 26 (5.1%) | 3.33 | 2.10–5.29 | 0.000 | B | 2.10 | 1.31–3.38 | 0.002 |
| Systemic hypertension | 138 (26.9%) | 2.02 | 1.60–2.56 | 0.000 | B, C | 1.33 | 1.03–1.72 | 0.028 |
| Peripheral vascular diseasea(1pt) | 18 (3.5%) | 1.86 | 1.06–3.25 | 0.030 | ||||
| Peripheral artery disease grade I | 12 (2.3%) | 1.24 | 0.58–2.62 | 0.579 | ||||
| Aortic dissection | 3 (0.6%) | 5.82 | 1.86–18.26 | 0.003 | ||||
| Aortic ectasia | 4 (0.8%) | 2.92 | 1.08–7.86 | 0.034 | ||||
| Cerebrovascular diseasea(1pt) | 11 (2.1%) | 0.97 | 0.46–2.04 | 0.927 | ||||
| Hemiplegiaa(2pt) | 0 | |||||||
| Epilepsy | 6 (1.2%) | 1.08 | 0.45–2.61 | 0.866 | ||||
| Dementiaa(1pt) | 0 | |||||||
| Connecstive tissue diseasea(1pt) | 22 (4.3) | 0.89 | 0.52–1.53 | 0.683 | ||||
| Rheumatoid arthritis | 10 (1.9%) | 1.66 | 0.82–3.36 | 0.156 | ||||
| Scleroderma | 6 (1.2%) | 0.44 | 0.14–1.39 | 0.163 | ||||
| Liver disease milda(1pt) | 78 (15.2%) | 1.17 | 0.85–1.60 | 0.350 | ||||
| Liver disease moderatea(3pt) | 12 (2.3%) | 1.49 | 1.19–1.87 | 0.000 | A, B, C | 1.41 | 1.12–1.77 | 0.004 |
| Peptic ulcer diseasea(1pt) | 18 (3.5%) | 2.49 | 1.48–4.19 | 0.001 | A, B, C | 1.78 | 1.00–3.24 | 0.040 |
| Gastroesophageal reflux | 147 (28.7%) | 1.67 | 1.32–2.12 | 0.000 | A, B, C | 1.28 | 1.00–1.65 | 0.023 |
| Barret oesophagus | 17 (3.3%) | 1.44 | 0.81–2.57 | 0.217 | ||||
| Chronic pulmonary diseasea(1pt) | 513 (100.0%) | |||||||
| Diabetes milda(1pt) | 90 (17.5%) | 0.85 | 0.64–1.13 | 0.262 | ||||
| Diabetes end-organ damagea(2pt) | 8 (1.6%) | 1.45 | 1.01–2.07 | 0.043 | A, B, C | 1.59 | 1.11–2.28 | 0.012 |
| Moderate or severe renal diseasea(2pt) | 61 (11.9%) | 1.64 | 1.41–1.92 | 0.000 | A, B, C | 1.38 | 1.18–1.62 | 0.000 |
| BMI (median, range) | 20.8 (13.1–38.1) | 1.05 | 1.03–1.07 | 0.000 | ||||
| 30.0–34.9 | 28 (5.5%) | 1.42 | 0.92–2.19 | 0.112 | ||||
| ≥35 | 4 (0.8%) | 3.09 | 1.15–8.31 | 0.025 | ||||
| <18.5 | 142 (27.7%) | 0.77 | 0.61–0.98 | 0.031 | ||||
| Osteoporosis | 178 (34.7%) | 1.52 | 1.22–1.89 | 0.000 | A, B, C | 1.52 | 1.21–1.92 | 0.000 |
| Diverticulosis | 65 (12.7%) | 2.02 | 1.48–2.75 | 0.000 | A, B, C | 1.42 | 1.01–2.00 | 0.043 |
| Morbus Crohn/Colitis ulcerosa | 6 (1.2%) | 1.21 | 0.39–3.78 | 0.743 | ||||
| Cholecystolithiasis | 30 (5.8%) | 1.23 | 0.78–1.93 | 0.373 | ||||
| Pre-transplant critical situation (e.g., MV, ECMO, ICU) | 56 (10.9%) | 1.53 | 1.08–2.17 | 0.017 | ||||
| Pre-transplant ECMO | 34 (6.6%) | 1.51 | 0.97–2.35 | 0.071 | ||||
| Lymphomaa(2pt) | 6 (1.2%) | 0.75 | 0.43–1.33 | 0.331 | ||||
| Leukemiaa(2pt) | 1 (0.2%) | 2.38 | 0.89–6.38 | 0.085 | ||||
| Tumora(2pt) | 24 (4.7%) | 1.18 | 0.92–1.50 | 0.198 | ||||
| Metastatic solid tumora(6pt) | 0 | |||||||
| AIDSa(6pt) | 0 | |||||||
| aCharlson-Deyo-Index pt (median, range) | 2 (1–8) | 1.37 | 1.26–1.48 | 0.000 | ||||
| 1 | 142 (27.7%) | D | Ref | |||||
| 2 | 166 (32.4%) | 1.56 | 1.18–2.05 | 0.002 | ||||
| 3 | 100 (19.5%) | 1.65 | 1.19–2.30 | 0.003 | ||||
| 4 | 54 (10.5%) | 3.08 | 2.11–4.50 | 0.000 | ||||
| ≥5 | 51 (9.9%) | 4.10 | 2.76–6.09 | 0.000 | ||||
| Transplant and Donor Characteristics | ||||||||
| Era 1992–2000 vs. 2001–2019 | 98 (19.1%) | 1.31 | 1.00–1.71 | 0.051 | ||||
| Era 1992–2008 vs. 2009–2019 | 247 (48.1%) | 1.22 | 0.97–1.54 | 0.093 | ||||
| Unilateral Transplantation | 36 (7.0%) | 2,01 | 1.41–2.87 | 0.000 | A, B, C, D | 2.68 | 1.85–3.87 | 0.000 |
| Re-Transplantation | 23 (4.5%) | 2.41 | 1.53–3.80 | 0.000 | ||||
| Intra-operative ECMO use | 241 (47.0%) | 1.40 | 1.14–1.73 | 0.002 | ||||
| CMV high risk | 131 (25.5%) | 1.01 | 0.79–1.28 | 0.961 | ||||
| Zurich Donor Score, median (range) | 3 (0–12) | 1.13 | 1.09–1.18 | 0.000 | A, B, C, D | 1.10 | 1.06–1.15 | 0.000 |
| DCD | 28 (5.5%) | 0.90 | 0.50–1.61 | 0.718 | ||||
| EVLP | 10 (1.9%) | 0.78 | 0.32–1.88 | 0.575 | ||||
| PGD3 at T72 | 79 (15.4%) | 2.07 | 1.58–2.70 | 0.000 | ||||
Pre-transplant recipient characteristics for survival.
Variables and points (pt) of Charlson-Deyo-Index.
Abbreviations: AIDS, acquired immune deficiency syndrome; BMI, body mass index; CI, confidence interval; CMV, cytomegalo virus; DCD, lung donation after circulatory death; HR, hazard ratio; MV, mechanical ventilation; ECMO, extracorporeal membrane oxygenation; EVLP, ex vivo lung perfusion; FEV1, forced expiratory volume in 1 s; ICU, intensive care unit; mPAP, mean pulmonary artery pressure; OR, odds ratio; PGD, primary graft dysfunction py, pack years.
TABLE 2
| N = 79/507 | Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | Model | OR | 95% CI | p | ||
| Recipient Characteristics | ||||||||
| Age (median, range) | 48 (18–68) | 1.01 | 0.99–1.02 | 0.586 | ||||
| Sex male | 38 (48.1%) | 0.81 | 0.50–1.31 | 0.398 | ||||
| Diagnosis | ||||||||
| Cystic fibrosis | 18 (22.8%) | 0.62 | 0.35–1.09 | 0.096 | ||||
| Idiopathic pulmonary arterial hypertension | 14 (17.7%) | 6.36 | 0.29–13.97 | 0.000 | ||||
| Emphysema | 8 (10.1%) | 0.22 | 0.10–0.46 | 0.000 | ||||
| Idiopathic pulmonary fibrosis | 28 (35.4%) | 2.35 | 1.40–3.96 | 0.001 | ||||
| Other | 18 (22.8%) | |||||||
| Smoking (pack years) (median, range) | 0 (0–80) | 0.98 | 0.97–1.00 | 0.015 | ||||
| >20py | 21 (26.6%) | 0.58 | 0.34–1.00 | 0.048 | ||||
| Waitlist (days) (median, range) | 39 (11–88) | 1.00 | 1.00–1.00 | 0.274 | ||||
| Recipient Comorbidities | ||||||||
| Any coronary artery disease | 5 (6.3%) | 0.48 | 0.19–1.24 | 0.128 | ||||
| Myocardial infarctiona(1pt) | 1 (1.3%) | 0.90 | 0.11–7.59 | 0.924 | ||||
| Postinterventional coronary disease (stent) | 2 (2.5%) | 0.83 | 0.18–3.75 | 0.808 | ||||
| Coronary disease mild | 3 (3.8%) | 0.38 | 0.12–1.27 | 0.117 | ||||
| Congestive heart failurea(1pt) | 64 (81.0%) | 5.00 | 2.76–9.06 | 0.000 | A | 4.28 | 2.34–7.83 | 0.000 |
| Right heart failure | 63 (79.7%) | 4.79 | 2.68–8.57 | 0.000 | C | 2.47 | 1.28–4.80 | 0.007 |
| mPAP (median, range) | 35 (20–80) | 1.04 | 1.03–1.06 | 0.000 | B | 2.15 | 1.12–4.15 | 0.022 |
| >25 mmHg | 62 (78.5%) | 4.32 | 2.44–7.62 | 0.000 | ||||
| Left heart failure | 4 (5.1%) | 3.21 | 0.92–11.23 | 0.068 | ||||
| Chronic atrial fibrillation | 6 (7.6%) | 1.87 | 0.72–4.87 | 0.199 | ||||
| Systemic hypertension | 25 (31.6%) | 1.31 | 0.78–2.20 | 0.315 | ||||
| Peripheral vascular diseasea(1pt) | 2 (2.5%) | 0.67 | 0.15–2.97 | 0.597 | ||||
| Peripheral artery disease grade I | 0 | — | ||||||
| Aortic dissection | 1 (1.3%) | 2.73 | 0.25–30.48 | 0.414 | ||||
| Aortic ectasia | 1 (1.3%) | 1.82 | 0.19–17.69 | 0.607 | ||||
| Cerebrovascular diseasea(1pt) | 0 | — | ||||||
| Hemiplegiaa(2pt) | 0 | — | ||||||
| Epilepsy | 0 | — | ||||||
| Dementiaa(1pt) | 0 | — | ||||||
| Connective tissue diseasea(1pt) | 7 (8.9%) | 2.68 | 1.06–6.79 | 0.038 | ||||
| Rheumatoid arthritis | 3 (3.8%) | 2.37 | 0.60–9.38 | 0.218 | ||||
| Scleroderma | 3 (3.8%) | 5.59 | 1.11–28.22 | 0.037 | ||||
| Liver disease milda(1pt) | 14 (17.7%) | 1.25 | 0.66–2.36 | 0.495 | ||||
| Liver disease moderatea(3pt) | 2 (2.5%) | 1.07 | 0.64–1.79 | 0.810 | ||||
| Peptic ulcer diseasea(1pt) | 1 (1.3%) | 0.31 | 0.04–2.36 | 0.258 | ||||
| Gastroesophageal reflux | 22 (27.8%) | 0.99 | 0.58–1.69 | 0.973 | ||||
| Barret oesophagus | 0 | — | ||||||
| Chronic pulmonary diseasea(1pt) | 79 (100.0%) | — | ||||||
| Diabetes milda(1pt) | 12 (15.2%) | 0.83 | 0.43–1.61 | 0.580 | ||||
| Diabetes end-organ damagea(2pt) | 2 (2.5%) | 1.48 | 0.65–3.40 | 0.352 | ||||
| Moderate or severe renal diseasea(2pt) | 11 (13.9%) | 1.12 | 0.79–1.59 | 0.532 | ||||
| BMI (median, range) | 22.8 (14.7–36.0) | 1.09 | 1.04–1.15 | 0.001 | ||||
| ≥30.0 | 13 (16.5%) | 5.42 | 2.47–11.91 | 0.000 | A, B, C, D | 4.27 | 1.88–9.68 | 0.001 |
| ≥35 | 2 (2.5%) | 5.53 | 0.77–39.87 | 0.090 | ||||
| <18.5 | 19 (24.1%) | 0.80 | 0.46–1.40 | 0.441 | ||||
| Osteoporosis | 33 (41.8%) | 1.43 | 0.88–2.33 | 0.153 | ||||
| Diverticulosis | 15 (19.0%) | 1.81 | 0.96–3.43 | 0.067 | ||||
| Morbus Crohn/Colitis ulcerosa | 0 | — | ||||||
| Cholecystolithiasis | 3 (3.8%) | 0.59 | 0.17–1.98 | 0.390 | ||||
| Pre-transplant critical situation (e.g., MV, ECMO, ICU) | 14 (17.7%) | 2.15 | 1.11–4.18 | 0.024 | ||||
| Pre-transplant ECMO | 10 (12.7%) | 2.81 | 1.27–6.22 | 0.011 | ||||
| Lymphomaa(2pt) | 1 (1.3%) | 1.04 | 0.35–3.07 | 0.941 | ||||
| Leukemiaa(2pt) | 0 | — | ||||||
| Tumora(2pt) | 3 (3.8%) | 0.90 | 0.48–1.67 | 0.732 | ||||
| Metastatic solid tumora(6pt) | 0 | — | ||||||
| AIDSa(6pt) | 0 | — | ||||||
| aCharlson-Deyo-Index pt (median, range) | 2 (1–6) | 1.22 | 1.04–1.45 | 0.017 | ||||
| 1 | D | Ref | ||||||
| 2 | 3.42 | 1.54–7.57 | 0.002 | |||||
| 3 | 2.45 | 1.01–5.91 | 0.047 | |||||
| ≥4 | 3.75 | 1.60–8.77 | 0.002 | |||||
| Transplant and Donor Characteristics | ||||||||
| Era 1992–2000 vs. 2001–2019 | 11 (13.9%) | 1.58 | 0.80–3.11 | 0.188 | ||||
| Era 1992–2008 vs. 2009–2019 | 44 (55.7%) | 0.71 | 0.44–1.15 | 0.166 | ||||
| Unilateral Transplantation | 3 (3.8%) | 0.49 | 0.15–1.64 | 0.245 | ||||
| Re-Transplantation | 1 (1.3%) | 0.26 | 0.04–1.98 | 0.194 | ||||
| Intra-operative ECMO use | 60 (75.9%) | 1.52 | 2.61–7.84 | 0.000 | A, B, C | 2.93 | 1.56–5.53 | 0.001 |
| CMV high risk | 17 (21.5%) | 0.76 | 0.42–1.35 | 0.341 | ||||
| Zurich Donor Score, median (range) | 3 (0–11) | 1.14 | 1.04–1.24 | 0.003 | A, B, C, D | 1.11 | 1.01–1.21 | 0.028 |
| DCD | 3 (3.8%) | 0.64 | 0.19–2.16 | 0.469 | ||||
| EVLP | 2 (2.5%) | 0.73 | 0.15–3.52 | 0.698 | ||||
Pre-transplant recipient characteristics for PGD3 on day 3.
Variables and points (pt) of Charlson-Deyo-Index.
Abbreviations: AIDS, acquired immune deficiency syndrome; BMI, body mass index; CI, confidence interval; CMV, cytomegalo virus; DCD, lung donation after circulatory death; HR, hazard ratio; MV, mechanical ventilation; ECMO, extracorporeal membrane oxygenation; EVLP, ex vivo lung perfusion; FEV1, forced expiratory volume in 1 s; ICU, intensive care unit; mPAP, mean pulmonary artery pressure; OR, odds ratio; PGD, primary graft dysfunction py, pack years.
TABLE 3
| N = 266/513 | Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | Model | HR | 95% CI | p | ||
| Recipient Characteristics | ||||||||
| Age (median, range) | 51 (18–70) | 1.01 | 1.00–1.02 | 0.002 | ||||
| Sex male | 143 (53.8%) | 1.11 | 0.88–1.41 | 0.380 | ||||
| Diagnosis | ||||||||
| Cystic fibrosis | 69 (25.9%) | 0.69 | 0.53–0.91 | 0.007 | ||||
| Idiopathic pulmonary arterial hypertension | 13 (4.9%) | 0.82 | 0.48–1.40 | 0.470 | ||||
| Emphysema | 86 (32.3%) | 1.17 | 0.91–1.50 | 0.230 | ||||
| Idiopathic pulmonary fibrosis | 64 (24.1%) | 1.38 | 1.03–1.86 | 0.031 | A, B, D | 1.44 | 1.07–1.95 | 0.017 |
| Other | ||||||||
| Smoking (pack years) (median, range) | 4 (0–120) | 1.01 | 1.00–1.01 | 0.001 | A, B, C, D | 1.48 | 1.16–1.91 | 0.002 |
| >20py | 112 (42.1%) | |||||||
| Waitlist (days) (median, range) | 150.5 (0–1378) | 1.00 | 1.00–1.00 | 0.820 | ||||
| Recipient Comorbidities | ||||||||
| Any coronary artery disease | 32 (12.0%) | 1.33 | 0.89–1.98 | 0.160 | ||||
| Myocardial infarctiona(1pt) | 2 (0.8%) | 0.57 | 0.12–2.66 | 0.470 | ||||
| Postinterventional coronary disease (stent) | 8 (3.0%) | 0.95 | 0.41–2.18 | 0.900 | ||||
| Coronary disease mild | 24 (9.0%) | 1.48 | 0.95–2.30 | 0.080 | ||||
| Congestive heart failurea(1pt) | 142 (53.4%) | 1.30 | 1.03–1.64 | 0.030 | A | 1.27 | 1.00–1.16 | 0.053 |
| Right heart failure | 140 (52.6%) | 1.31 | 1.04–1.66 | 0.023 | B | 1.24 | 0.98–1.58 | 0.078 |
| mPAP (median, range) | 32 (20–80) | 1.01 | 1.00–1.02 | 0.038 | C | 1.23 | 0.97–1.57 | 0.092 |
| >25 mmHg | 141 (53.0%) | |||||||
| Left heart failure | 6 (2.3%) | 1.10 | 0.41–2.93 | 0.850 | ||||
| Chronic atrial fibrillation | 9 (3.4%) | 0.67 | 0.33–1.38 | 0.280 | ||||
| Systemic hypertension | 74 (27.8%) | 1.29 | 0.97–1.70 | 0.077 | ||||
| Peripheral vascular diseasea(1pt) | 5 (1.9%) | 0.50 | 0.20–1.27 | 0.140 | ||||
| Peripheral artery disease grade I | 2 (0.8%) | 0.29 | 0.07–1.19 | 0.085 | ||||
| Aortic dissection | 1 (0.4%) | 0.61 | 0.07–5.50 | 0.660 | ||||
| Aortic ectasia | 3 (1.1%) | 2.13 | 0.62–7.27 | 0.230 | ||||
| Cerebrovascular diseasea(1pt) | 6 (2.3%) | 1.32 | 0.62–2.81 | 0.460 | ||||
| Hemiplegiaa(2pt) | 0 | |||||||
| Epilepsy | 5 (1.9%) | 1.92 | 0.90–4.07 | 0.089 | A, B, C, D | 2.34 | 1.06–5.19 | 0.036 |
| Dementiaa(1pt) | 0 | |||||||
| Connective tissue diseasea(1pt) | 15 (5.6%) | 1.44 | 0.87–2.39 | 0.150 | ||||
| Rheumatoid arthritis | 8 (3.0%) | 2.24 | 1.07–4.72 | 0.033 | ||||
| Scleroderma | 4 (1.5%) | 1.10 | 0.46–2.67 | 0.830 | ||||
| Liver disease milda(1pt) | 29 (10.9%) | 0.74 | 0.49–1.10 | 0.130 | ||||
| Liver disease moderatea(3pt) | 4 (1.5%) | 0.87 | 0.62–1.22 | 0.410 | ||||
| Peptic ulcer diseasea(1pt) | 6 (2.3%) | 0.73 | 0.28–1.85 | 0.500 | ||||
| Gastroesophageal reflux | 71 (26.7%) | 1.05 | 0.80–1.38 | 0.740 | ||||
| Barret oesophagus | 9 (3.4%) | 1.10 | 0.57–2.12 | 0.780 | ||||
| Chronic pulmonary diseasea(1pt) | 266 (100.0%) | |||||||
| Diabetes milda(1pt) | 41 (15.4%) | 0.82 | 0.59–1.13 | 0.230 | ||||
| Diabetes end-organ damagea(2pt) | 5 (1.9%) | 1.17 | 0.72–1.90 | 0.520 | ||||
| Moderate or severe renal diseasea(2pt) | 25 (9.4%) | 0.91 | 0.72–1.14 | 0.400 | ||||
| BMI (median, range) | 21.1 (13.1–36.0) | 1.05 | 1.02–1.08 | 0.000 | ||||
| ≥30.0 | 18 (6.8%) | |||||||
| ≥35 | 2 (0.8%) | |||||||
| <18.5 | 52 (27.2%) | |||||||
| Osteoporosis | 94 (35.3%) | 1.15 | 0.89–1.49 | 0.270 | ||||
| Diverticulosis | 36 (13.5%) | 1.27 | 0.89–1.82 | 0.190 | ||||
| Morbus Crohn/Colitis ulcerosa | 1 (0.4%) | 0.35 | 0.05–2.51 | 0.300 | ||||
| Cholecystolithiasis | 13 (4.9%) | 0.80 | 0.47–1.36 | 0.410 | ||||
| Pre-transplant critical situation (e.g., MV, ECMO, ICU) | 20 (7.5%) | 0.68 | 0.42–1.09 | 0.110 | ||||
| Pre-transplant ECMO | 11 (4.1%) | 0.64 | 0.33–1.36 | 0.180 | ||||
| Lymphomaa(2pt) | 2 (0.8%) | 0.70 | 0.36–1.36 | 0.290 | ||||
| Leukemiaa(2pt) | 0 | |||||||
| Tumora(2pt) | 11 (4.1%) | 0.95 | 0.70–1.29 | 0.730 | ||||
| Metastatic solid tumora(6pt) | 0 | |||||||
| AIDSa(6pt) | 0 | |||||||
| aCharlson-Deyo-Index pt (median, range) | 2 (1–6) | 0.96 | 0.88–1.05 | 0.330 | ||||
| 1 | 76 (28.6%) | D | Ref | |||||
| 2 | 99 (37.2%) | 1.29 | 0.98–1.71 | 0.074 | ||||
| 3 | 50 (18.8%) | 1.04 | 0.72–1.49 | 0.840 | ||||
| 4 | 20 (7.5%) | 0.80 | 0.53–1.20 | 0.270 | ||||
| ≥5 | 21 (7.9%) | 1.28 | 0.95–1.72 | 0.100 | ||||
| Transplant and Donor Characteristics | ||||||||
| Era 1992–2000 vs. 2001–2019 | 50 (18.8%) | 1.43 | 1.08–1.90 | 0.011 | D | 1.28 | 0.95–1.72 | 0.100 |
| Era 1992–2008 vs. 2009–2019 | 146 (54.9%) | 1.01 | 0.80–1.28 | 0.920 | ||||
| Unilateral Transplantation | 15 (5.6%) | 0.71 | 0.41–1.23 | 0.220 | ||||
| Re-Transplantation | 7 (2.6%) | 0.52 | 0.23–1.19 | 0.120 | ||||
| Intra-operative ECMO use | 126 (47.4%) | 1.23 | 0.97–1.56 | 0.092 | ||||
| CMV high risk | 76 (28.6%) | 1.27 | 0.98–1.66 | 0.075 | A, B, C, D | 1.32 | 1.01–1.74 | 0.026 |
| Zurich Donor Score, median (range) | 3 (0–11) | 1.06 | 1.02–1.11 | 0.007 | A, B, C, D | 1.05 | 1.00–1.10 | 0.048 |
| DCD | 11 (4.1%) | 0.95 | 0.51–1.77 | 0.880 | ||||
| EVLP | 4 (1.5%) | 0.95 | 0.31–2.93 | 0.930 | ||||
| PGD3 at T72 | 43/(16.2%) | 1.19 | 0.84–1.68 | 0.340 | ||||
Pre-transplant recipient characteristics for onset of CLAD-3.
Variables and points (pt) of Charlson-Deyo-Index.
Abbreviations: AIDS, acquired immune deficiency syndrome; BMI, body mass index; CI, confidence interval; CMV, cytomegalo virus; DCD, lung donation after circulatory death; HR, hazard ratio; MV, mechanical ventilation; ECMO, extracorporeal membrane oxygenation; EVLP, ex vivo lung perfusion; FEV1, forced expiratory volume in 1 s; ICU, intensive care unit; mPAP, mean pulmonary artery pressure; OR, odds ratio; PGD, primary graft dysfunction py, pack years.
Definition of Comorbidities
The comorbidities in the CDI were defined by relying mostly on the original publication (4). In our selection program, all candidates with risk factors for coronary artery disease or aged ≥50 years old were evaluated by coronary angiogram. Congestive heart failure contains right or left heart failure or a combination of both. Right heart failure was defined as a mean pulmonary artery pressure (mPAP) >25 mmHg combined with echocardiographic evidence of right ventricular dysfunction (ventricular hypertrophy, moderate valve insufficiency, pericardial effusion) and/or signs of secondary liver or kidney dysfunction; left heart failure as having a reduced left ventricular ejection fraction <40%. Peripheral vascular disease includes aortic aneurysm, aortic ectasia and peripheral arterial disease grade I-IV. Cerebrovascular disease is defined as history of stroke with residual neurological deficit or transient ischemic attack. Connective tissue disease includes diagnosis of systemic lupus, rheumatoid arthritis, scleroderma, or seronegative spondyloarthropathy. Mild diabetes mellitus is type 1 and type 2 requiring medication, excluding dietary-controlled diabetes. For diabetes with end-organ damage renal, ophthalmic or neurological manifestations are required. Mild liver disease is defined as no portal hypertension with elevated liver enzymes more than three times the upper limit of normal. Moderate liver disease includes forms of fibrosis or cirrhosis causing portal hypertension with elevated liver enzymes. Moderate or severe renal disease includes glomerular filtration rate (eGFR) ≤60 ml/min/1.73 m2 or acute renal replacement therapy. Tumor means a history of malignancy, excluding non-melanoma skin cancer. Further comorbidities were selected based on the 2014 and 2021 ISHLT consensus statement (1) and availability. Thereby, systemic hypertension was defined as without treatment ≥140/90 mmHg; critical or unstable condition such as mechanical ventilation (MV), extracorporeal membrane oxygenation (ECMO) or other reasons requiring pre-operative ICU; and osteoporosis as bone density with T-score below −2.5. To screen for diverticulosis and other colon disorders, candidates ≥50 years of age (for cystic fibrosis ≥40 years) were evaluated by colonoscopy. Gastroscopy was performed in all candidates with history of gastrointestinal symptoms or age ≥50 years. Gastroesophageal reflux disease was diagnosed predominantly on symptoms or endoscopic or radiological evidence, rarely on manometry and pH-metry testing.
Outcomes
The outcomes were PGD Grade-3 at 72 h, CLAD Grade-3 and survival after lung transplantation. PGD3-T72 is defined as PaO2/FiO2-ratio <200 mmHg and the presence of diffuse parenchymal infiltrates in the allograft on chest radiograph at 72 h after transplantation (9). As the definition was established in 2005, earlier cases were retrospectively analyzed by X-ray, ventilation curve and arterial blood gases. CLAD-3 is defined as a persistent decline of forced expiratory volume in 1 s (FEV1) ≤50% from baseline and an obstructive or restrictive physiology after exclusion of other causes (10).
Definition of Donor and Era Variables
To consider the impact of donor factors, the Zurich-Donor-Score (11) was used. This score estimates the quality of donor lungs, based on 5 extended donor criteria: age, diabetes mellitus, smoking history, pulmonary infection, and ratio of partial pressure of arterial oxygen to inspired oxygen fraction. Due to change in induction and immunosuppression (Anti-thymocyte globuline to Basiliximab) therapy in 2000, this era effect was tested. Other arbitrary defined models splitting in two or three different eras of similar case size or years of transplant did not show any significant differences in survival.
Statistical Methods
Statistical analysis was performed with IBM SPSS version 26 (SPSS IBM, Armonk, New York, USA) and R (Version 4.0.5, Vienna, Austria). Continuous data were compared using the Mann–Whitney test and categorical variables compared using the v2 test or the Fisher’s exact test for expected frequencies <5. Kaplan-Meier method was used to estimate survival as well as time to CLAD-3. The log-rank test compared survival curves. Cox regression was used to assess risk factors for mortality. Cox regression for CLAD-3 was adjusted for the competing factor of death by the Fine Gray methodology. Logistic regression was used to assess factors for PGD3-T72. First, every variable was checked with a univariate (enter) model. Variables with a p-value < 0.2 (12) were tested in a multivariate stepwise backward Cox regression model or linear regression model, respectively. The number of factors introduced into the final multivariable model was calculated by considering sample size and number of occurring events (13). To confirm that variables show a stable significance, they had to be frequent in number. Linear regression was used to test collinearity between variables. A variance inflation factor >5 and a tolerance <0.2 was defined as indicating a collinearity problem. Different final multivariate models are provided to bypass variables with statistical or clinical collinearity. In general, a p-value < 0.05 was considered to be the threshold for statistical significance.
The local research ethics review committee approved the study (KEK-Nr.2019-00873).
Results
In our study population, there were 513 adult recipients who underwent lung transplantation between 1992 and 2019. Of these, 353 recipients (68.8%) died, 266 (51.9%) developed CLAD-3 and 79 (15.4%) PGD3-T72. Median follow-up time was 12.7 years. No loss to follow-up occurred. Half of the transplants were performed in the era 1992–2008 and showed a trend of better survival than the era 2009–2019 (median survival 8.4 vs. 5.9 years, respectively, log-rank = 0.092). The same was observed for onset of CLAD-3 (median 7.6 vs. 5.5 years, respectively, log-rank = 0.121). In line with these trends, donor marginality measured by ZDS (mean 2.8 vs. 4.0 points, p < 0.001) and the recipient comorbidity burden measured by CDI (mean 2.2 vs. 2.7 points, p < 0.001) increased significantly in the second era. Figure 1 shows the detailed increase of the CDI score burden over the study period. In the earlier era a trend of more PGD3-T72 occurred (17.8% vs. 13.2%, respectively, p = 0.144).
FIGURE 1
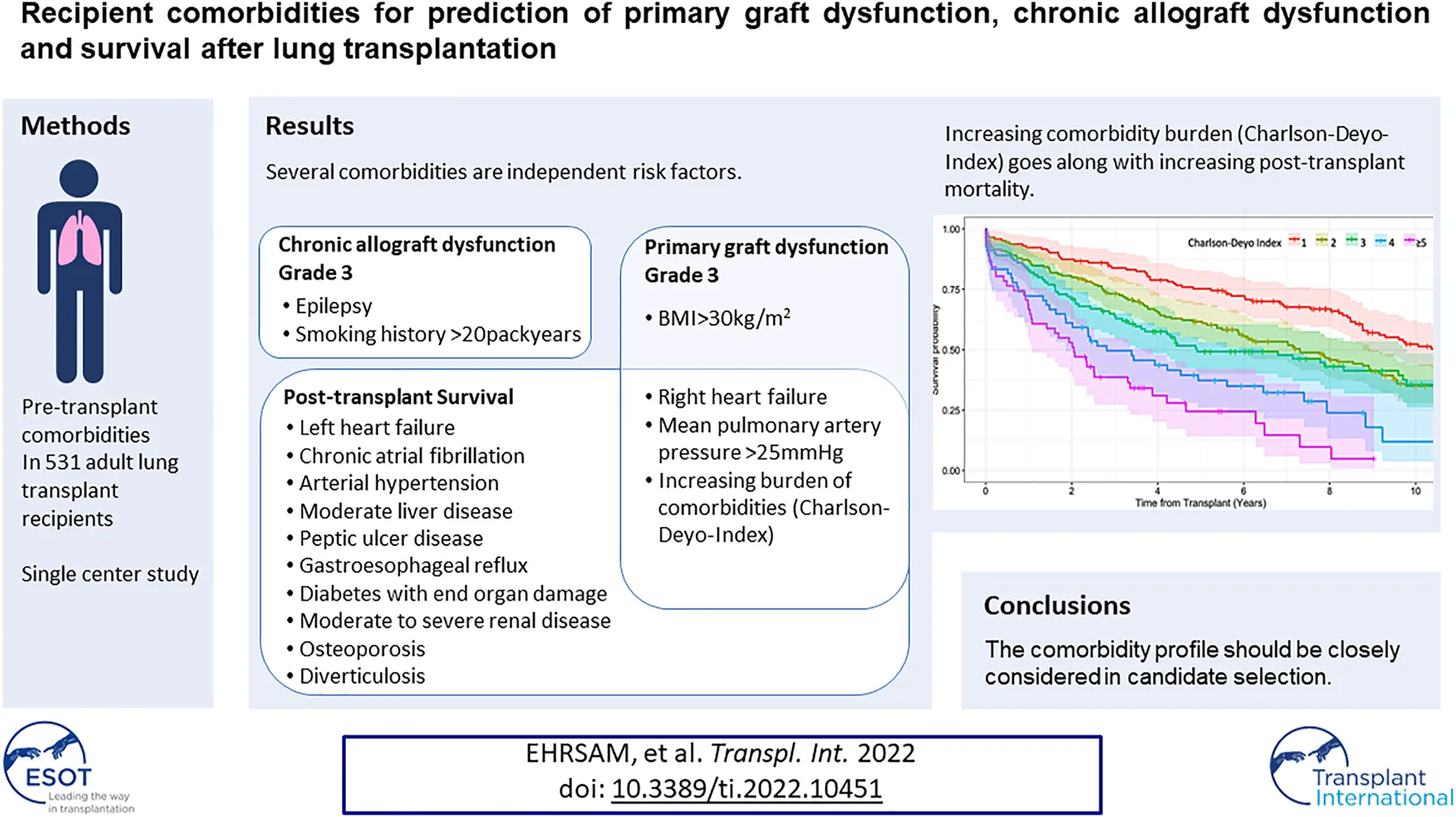
Seventy two percent of the recipients had at least one comorbidity represented in the CDI, beside of the always present underlying chronic pulmonary disease which accounts for an extra point. As illustrated in the Kaplan-Meier survival curve of Figure 2A, an increasing number or severity of comorbidities in the CDI was associated with significantly poorer survival, except that a score of 2 points was comparable to a score of 3 points (log-rank = 0.776). The median survival for a CDI score of 1, 2, 3, 4 and ≥5 points was 10.5, 7.3, 4.9, 2.8, and 2.1 years, respectively.
FIGURE 2
For the overall population, detailed descriptive statistics of recipient-, donor-, intra-operative characteristics are shown in Table 1. The most frequent underlining diseases were cystic fibrosis (30%) and emphysema (30%). The most frequent comorbidity was congestive heart failure (52%) including in 98% of these cases right heart failure all with an mPAP >25 mmHg. The next most frequent comorbidities were osteoporosis (35%), gastroesophageal reflux (29%), systemic hypertension (27%), mild diabetes (18%), mild liver disease (15%), diverticulosis (13%) and moderate to severe renal disease (12%).
Risk Factors for Survival
All comorbidities listed in Table 1 were assessed in univariable and if applicable in multivariable risk analysis. In multivariable Cox regression (Table 1, Model A), moderate liver disease, peptic ulcer disease, gastroesophageal reflux, diabetes with end-organ damage, moderate to severe renal disease, osteoporosis, diverticulosis, and congestive heart failure were independent risk factors for mortality, beside of increasing age, increasing ZDS and unilateral lung transplantation. The subgroups of left heart failure and right heart failure as well as mPAP >25 mmHg, chronic atrial fibrillation and systemic hypertension were also multivariate risk factors for mortality when independently analyzed from congestive heart failure (Table 1, Model B, C). Of note, the underlying lung diseases were no multivariable risk factors in the models, after introducing comorbidities. The same effect was found for re-transplantation, pre-transplant critical situation, ECMO as bridge to transplantation and intraoperative ECMO use.
The accumulation of comorbidities with CDI in the multivariable model (Table 1, Model D) showed an even better performance for survival estimates than the unadjusted Kaplan-Meier curves (Figure 2A).
Risk Factors for PGD3-T72
Recipient-, donor-, intra-operative characteristics for those transplantations where PGD3-T72 occurred are listed in Table 2. In this subpopulation, the underlying diagnosis of idiopathic pulmonary fibrosis (35%, p = 0.001) and idiopathic pulmonary arterial hypertension (18%, p < 0.001) were significantly higher represented. The percentage of congestive heart failure (81%, p < 0.001), a mPAP >25 mmHg (79%, p < 0.001), ECMO as bridge to transplantation (13%, p = 0.019), intraoperative ECMO use (76%, p < 0.001), CDI (p = 0.006) and ZDS (p = 0.011) were also significantly higher than in the overall population.
In multivariable logistic regression congestive heart failure, a BMI>30kg/m2, an increasing ZDS and intraoperative ECMO use were independent risk factors for PGD3-T72 (Table 2, Model A). Additional analyses excluding congestive heart failure revealed, that mPAP >25 mmHg and right heart failure were also factors for PGD3-T72 (Table 2, Model B, C). The accumulation of comorbidities in the CDI was associated with the risk of PGD3-T72 but not in a linear increasing way with increasing scoring points (Table 2, Model D), likely due to the small sample size.
Risk Factors for Onset of CLAD-3
For the subpopulation of CLAD-3, recipient-, donor-, intra-operative characteristics are listed in Table 3. The CLAD-3 subpopulation was comparable to the overall population with respect to the underlying disease and variables of intraoperative procedure, but showed a trend to more marginal donor lungs in the ZDS (p = 0.097) and a significantly higher comorbidity burden in the CDI (p = 0.018).
Multivariate Cox regression revealed that the underling diagnosis of idiopathic pulmonary fibrosis, a smoking history of the recipient of >20 packyears, epilepsy, CMV high-risk constellation and an increasing ZDS were independent risk factors for onset of CLAD-3 (Table 3, Model A, B, C). Congestive heart failure, right heart failure and mPAP >25 mmHg were borderline risk factors (Table 3, Model A, B, C). The change in induction and immunosuppression in 2000 from Anti-thymocyte globuline to Basiliximab was a borderline risk factor (Table 3, Model D). Recipient age and PGD-3 were no risk factors for developing CLAD-3.
Moreover, the comorbidity burden estimated by CDI was not a multivariable risk factor for developing CLAD-3 (Table 3, Model D). This is in line with the Kaplan-Meier estimate, where onset of CLAD-3 was not gradually reduced by an increasing CDI (Figure 2B). The median time until onset of CLAD-3 for a CDI score of 1, 2, 3, 4 and ≥5 points was 8.4, 5.5, 5.9, 8.4, and 3.0 years, respectively.
Discussion
This study is the first detailed analysis of association between recipient comorbidities prior to transplantation and survival, PGD3-T72 and onset of CLAD-3 after lung transplantation. We show that several recipient comorbidities and their accumulation have a strong impact on post-transplant survival, and that some comorbidities also affect the development of PGD3-T72 and CLAD-3.
It is paramount to define the right time of listing and transplanting a candidate. On one hand, a limited life expectancy due to the lung disease is required to justifying the benefit over the risk of a lung transplantation. On the other hand, a prolonged time span until transplantation is often associated with developing a more extensive comorbidity profile. This problem is further aggravated by a demographic shift toward older candidates, who are per se more likely to be multi-morbid.
While lung transplantation may improve previously poor organ oxygenation and consecutively slow down the progression of many comorbidities, surgical complications and the side effects of the immunosuppression regime may worsen comorbidities considerably and even create new comorbidities over time.
In addition to respecting the ISHLT consensus document (1) for absolute contraindications, our center has been fairly liberal in the acceptance of candidates with reasonable comorbidities. Estimated by the CDI, 72% of our recipients had at least one comorbidity in addition to the underlying lung disease, providing ideal conditions for a thorough analysis.
Factors Associated With Survival
Among pretransplant recipient comorbidities, we identified right heart failure as an important risk factor affecting survival, PGD3-T72 and partially also CLAD-3. It was the most frequent comorbidity found in half of our cohort. Right heart failure and especially its approximative surrogate of pulmonary hypertension >25 mmHg were also risk factors for mortality in a single center study (14) and in the United Network for Organ Sharing (UNOS) Database in 3105 emphysema patients (15). Even though right heart failure may be partially to fully reversible after lung transplantation, pulmonary hypertension requires sometimes peri-operative extracorporeal membrane oxygenation (ECMO) implantation to avoid reperfusion edema which goes along with a variety of factors that can increase morbidity (16). One of the morbidities is PGD attributed to the systemic inflammatory response associated with the machine as well as its systemic anticoagulation requirements (17). In our cohort, intraoperative ECMO use was also an independent risk factor for developing PGD3-T72.
In our study, the few cases of left heart failure were also strongly associated with mortality. Previous reports about left heart failure are lacking, likely as it is widely considered a contraindication for transplantation (2).
Systemic hypertension was present in one fourth of our cohort. It was a risk factor for mortality, in line with a previous report in 821 pulmonary fibrosis recipients (18). Pretransplant systemic hypertension may aggravate differently after transplant because of the side effects of immunosuppression treatment with calcineurin inhibitors than in previously non-hypertensive recipients. This might lead to earlier end organ damage. Moreover, a meta-analysis (19) has shown that systemic hypertension was a risk factor for postoperative atrial arrhythmias and therefore had prognostic implications for length of hospital stay and overall survival.
Pretransplant atrial fibrillation increased the risk of adverse cardiovascular outcomes and longer hospital stay in a single-center study (20). In our study, pretransplant chronic atrial fibrillation was even an independent risk factor for mortality.
We identified diabetes mellitus with end-organ damage but not mild diabetes as a risk factor for mortality. This is in line with the findings of the University of Melbourne study (21) for poorly controlled glycemic controlled candidates. The ISHLT report even lists any stage of diabetes as a risk factor for 10-year mortality (22), including diabetes without end organ damage.
Renal disease may further aggravate in the peritransplant period mainly due to the immunosuppression regimen and fluid shifts after transplantation. Moderate to severe renal disease was an independent risk factor for mortality in our cohort. An eGFR of 60 ml/min/1.73m2 or less was also an independent risk factor for 1-year survival using UNOS data (23). And the ISHLT report lists recipient with a pre-transplant dialysis condition as a risk factor for 10-year mortality (22).
Currently, the impact of moderate liver disease is poorly understood because it has hardly been investigated so far. Although we found moderate liver disease to be a risk factor for mortality in our cohort, liver cirrhosis with or without portal hypertension did not have a negative impact on 5-year survival in 6 matched cystic fibrosis recipients in a previous study (24).
Gastroesophageal reflux was suggested to be associated with secondary aspiration contributing to acute rejection, pulmonary infection and CLAD and consecutive mortality (25). However, even though gastroesophageal reflux was an independent risk factor for mortality in our cohort, no risk association was found for development of PGD3-T72 and CLAD-3. A reason might be that several asymptomatic recipients were insufficiently screened in our program (26), preventing a correlation to PGD and CLAD. Another reason may be that we universally teach patients about anti-reflux measures (27).
Peptic ulcer disease was also a risk for mortality in our study. It is reported from small series to occur and reoccur after transplantation and may lead to intestinal perforation (28, 29).
The rate of developing acute diverticulitis from preexisting diverticulosis in immunosuppressed patients is significantly higher than in the general population (30). At our center, we reported an overall rate of diverticulitis of 4.5% after lung transplantation (31).
The prevalence of osteoporosis affected one third of our cohort and it was a significant risk factor for survival. Osteoporosis is in part reflected by preoperative steroid use which was a risk factor for 1-year survival in a study using UNOS data (23).
Neither mild nor post-interventional coronary disease were independent risk factors in our cohort, which is in line with previous studies (32, 33). Our cases with a history of myocardial infarction might have been too few in number or too highly selected to become an independent risk.
Multiple reports on other solid organ transplantations indicate that the presence of symptomatic peripheral vascular disease is one of the strongest predictors of mortality (34-36). In our study, a mild peripheral artery disease grade I seems to have minor impact on post-transplant survival. Previous aortic dissection and aortic ectasia appeared to be associated with post-transplant mortality in univariable analysis, but the limited number in our cohort did not justify further analysis.
We noted, that the underlying lung disease, a preoperative critical situation, and re-transplantation lost their strength as risk factors for mortality, when analyzed along with comorbidities. These variables may consecutively be regarded as surrogates for the comorbidity burden. For an optimal candidate selection, the focus should therefore lie on the comorbidity profile.
Factors Mainly Associated With PGD3-T72
In addition to right heart failure and mPAP >25 mmHg, described above, a BMI>30 kg/m2 was a strong risk factor for developing PGD3-T72 in our study. Pulmonary hypertension and BMI >25 were also reported as independent risk factors for PGD in a cohort of 7322 recipients (37) and in a meta-analysis (38). The mechanism of adipositas on PGD is not yet fully understood. It is likely caused by comorbidities associated with adipositas. This would also explain why adipositas was not a multivariable risk factor for mortality in our study.
Factors Mainly Associated With CLAD-3
This study is the first to detect epilepsy as a risk factor for CLAD-3. Some anti-epileptic medication show side effects on respiratory depression, increase oral and pulmonary secretions and even interstitial lung disease (39). Moreover, epilepsy might go along with an increased risk of aspiration leading to pneumonia, inflammation, and consecutive fibrotic alterations of the lung allograft. An additional risk for CLAD-3 was a previous smoking history of more than 20 packyears. We do not believe that the systemic damages caused by previous smoking is responsible for this effect, but the increased likelihood of being still exposed to a smoking environment or even due to smoking resumption (40). Another important aspect is the underlying disease in particular idiopathic pulmonary fibrosis. It was an independent factor for developing CLAD. The process may be due to the re-occurrence of the underlying disease in the allograft.
PGD was repeatedly associated with the risk of developing CLAD (41). However, we could not find such a correlation in our cohort. The detected borderline risk of a pre-transplant mPAP >25 mmHg might occasionally have caused de novo pulmonary hypertension and chronic lung edema and fibrosis of the lung allograft.
Charlson-Deyo-Index
An increasing comorbidity burden, estimated by the CDI, was well associated with an increasing risk for mortality. We showed that already one proportionally mild comorbidity in the CDI bears a significant risk on survival outcome. This should emphasize that a very careful selection of candidates considering comorbidities is crucial. However, we can not provide a recommendation based on our single-center analysis.
Our finding of CDI as a good predictor for survival is in line with multiple studies of other solid organ transplants (5-8). However, the Pittsburgh group (42) calculated the original Charlson Index for 748 lung transplant recipients and neither detected an association with in-hospital post-transplant complications nor an association with survival in a multivariate model. This might be due to an incomplete assessment of comorbidities, incomplete adjustment for confounders, and incorporation of recipient age in the score.
We detected several other comorbidities beyond the 18 comorbidity conditions represented in the CDI as important risk factors for survival. Thus, the addition of other comorbidities, a different weighing or sub-categorization may even improve the prediction of the CDI in the context of lung transplantation. This would have to be determined and proven in future studies.
The association of the comorbidity burden in the CDI was weaker for PGD3-T72 than for survival. Only one comorbidity of the CDI, congestive heart failure, was independently associated with onset of PGD3-T72 and borderline associated with onset of CLAD-3. Mechanisms of developing PGD and especially CLAD appear to rely more on a limited number of specific comorbidities, rather than on their quantity.
Limitations
This study has several limitations. It is a retrospective single-center study over more than 2 decades. The pre- and posttransplant treatment of some comorbidities might have changed over time. However, we could not detect an era effect in univariable and multivariable analyses. Some comorbidities might have been underrepresented in our study, which would have otherwise been important risk factors.
Conclusion
Our study identified several comorbidities that were associated with post-transplant survival, onset of PGD and CLAD. Based on our findings we consider the comorbidities mentioned in the current ISHLT-consensus document (2) as relative contraindications as valid risk factors for mortality after lung transplantation. The CDI may potentially be used for a more refined evaluation of multimorbid candidates.
Statements
Data availability statement
The datasets presented in this article are not readily available because Potential conflict with the Swiss Privacy Act, a federal law. The data that support the findings of this study are only available from the corresponding author, upon reasonable request. Requests to access the datasets should be directed to II, ilhan.inci@usz.ch.
Ethics statement
The studies involving human participants were reviewed and approved by the local research Ethics Review Committee (KEK-ZH-Nr.2019-00873). The patients/participants provided their written informed consent to participate in this study.
Author contributions
JE and II participated in conception and design of study. JE and II participated in acquisition of data. JE and ML participated in analysis of data. JE, MS, and II participated in interpretation of data. JE drafted the article. MS, IO, and II participated in revising the article critically. All authors approved to the version of the article to be published.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CDI, Charlson-Deyo-Index; CLAD, chronic lung allograft dysfunction; ISHLT, International Society for Heart and Lung Transplantation; mPAP, mean pulmonary artery pressure; UNOS, United Network for Organ Sharing; ZDS, Zurich Donor Score.
References
1.
WeillDBendenCCorrisPADarkJHDavisRDKeshavjeeSet alA Consensus Document for the Selection of Lung Transplant Candidates: 2014-An Update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant (2015) 34(1):1–15. 10.1016/j.healun.2014.06.014
2.
LeardLEHolmAMValapourMGlanvilleARAttawarSAversaMet alConsensus Document for the Selection of Lung Transplant Candidates: An Update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant (2021) 40(11):1349–79. 10.1016/j.healun.2021.07.005
3.
CharlsonMEPompeiPAlesKLMacKenzieCR. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J chronic Dis (1987) 40(5):373–83. 10.1016/0021-9681(87)90171-8
4.
DeyoRCherkinDCCiolMA. Adapting a Clinical Comorbidity index for Use with ICD-9-CM Administrative Databases. J Clin Epidemiol (1992) 45(6):613–9. 10.1016/0895-4356(92)90133-8
5.
GrossoGCoronaDMistrettaAZerboDSinagraNGiaquintaAet alPredictive Value of the Charlson Comorbidity index in Kidney Transplantation. Transplant Proc (2012) 44(7):1859–63. 10.1016/j.transproceed.2012.06.042
6.
LagingMKal-van GestelJAvan de WeteringJBetjesMGIJzermansJNWeimarWet alA High Comorbidity Score Should Not Be a Contraindication for Kidney Transplantation. Transplantation (2016) 100(2):400–6. 10.1097/tp.0000000000000973
7.
VolkMLHernandezJCLokASMarreroJA. Modified Charlson Comorbidity Index for Predicting Survival after Liver Transplantation. Liver Transpl (2007) 13(11):1515–20. 10.1002/lt.21172
8.
GrossoGdi FrancescoFVizziniGMistrettaAPaganoDEcheverriGJet alThe Charlson Comorbidity index as a Predictor of Outcomes in Liver Transplantation: Single-center Experience. Transplant Proc (2012) 44(5):1298–302. 10.1016/j.transproceed.2012.01.131
9.
ChristieJDCarbyMBagRCorrisPHertzMWeillDet alReport of the ISHLT Working Group on Primary Lung Graft Dysfunction Part II: Definition. A Consensus Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant (2005) 24(10):1454–9. 10.1016/j.healun.2004.11.049
10.
VerledenGMGlanvilleARLeaseEDFisherAJCalabreseFCorrisPAet alChronic Lung Allograft Dysfunction: Definition, Diagnostic Criteria, and Approaches to treatment―A Consensus Report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant (2019) 38(5):493–503. 10.1016/j.healun.2019.03.009
11.
EhrsamJPHeldUOpitzIInciI. A New Lung Donor Score to Predict Short and Long-Term Survival in Lung Transplantation. J Thorac Dis (2020) 12(10):5485–94. 10.21037/jtd-20-2043
12.
SauerbreiW. The Use of Resampling Methods to Simplify Regression Models in Medical Statistics. J R Statist Soc C (1999) 48(3):313–29. 10.1111/1467-9876.00155
13.
HosmerDWLemeshowSSturdivantRX. Applied Logistic Regression. New York, NY: Wiley (2000).
14.
KimCYParkJELeemAYSongJHKimSYChungKSet alPrognostic Value of Pre-transplant Mean Pulmonary Arterial Pressure in Lung Transplant Recipients: a Single-Institution Experience. J Thorac Dis (2018) 10(3):1578–87. 10.21037/jtd.2018.03.46
15.
HayesDJr.BlackSMTobiasJDMansourHMWhitsonBA. Prevalence of Pulmonary Hypertension and its Influence on Survival in Patients with Advanced Chronic Obstructive Pulmonary Disease Prior to Lung Transplantation. COPD: J Chronic Obstructive Pulm Dis (2016) 13(1):50–6. 10.3109/15412555.2015.1043425
16.
MagouliotisDETasiopoulouVSSvokosAASvokosKAZacharoulisD. Extracorporeal Membrane Oxygenation versus Cardiopulmonary Bypass during Lung Transplantation: a Meta-Analysis. Gen Thorac Cardiovasc Surg (2018) 66(1):38–47. 10.1007/s11748-017-0836-3
17.
HayangaJWAChanEGMusgroveKLeungAShigemuraNHayangaHK. Extracorporeal Membrane Oxygenation in the Perioperative Care of the Lung Transplant Patient. Semin Cardiothorac Vasc Anesth (2020) 24(1):45–53. 10.1177/1089253219896123
18.
MeyerDMEdwardsLBTorresFJessenMENovickRJ. Impact of Recipient Age and Procedure Type on Survival after Lung Transplantation for Pulmonary Fibrosis. Ann Thorac Surg (2005) 79(3):950–7. 10.1016/j.athoracsur.2004.08.076
19.
FanJZhouKLiSDuHCheG. Incidence, Risk Factors and Prognosis of Postoperative Atrial Arrhythmias after Lung Transplantation: a Systematic Review and Meta-Analysis. Interact Cardiovasc Thorac Surg (2016) 23(5):790–9. 10.1093/icvts/ivw208
20.
YerasiCRoySBOlsonMElnahasSKangPHashimiASet alOutcomes of Lung Transplant Recipients with Preoperative Atrial Fibrillation. Asian Cardiovasc Thorac Ann (2018) 26(2):127–32. 10.1177/0218492317754144
21.
HackmanKLSnellGIBachLA. Poor Glycemic Control Is Associated with Decreased Survival in Lung Transplant Recipients. Transplantation (2017) 101(9):2200–6. 10.1097/tp.0000000000001555
22.
ChambersDCCherikhWSHarhayMOHayesDJr.HsichEKhushKKet alThe International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-Sixth Adult Lung and Heart-Lung Transplantation Report-2019; Focus Theme: Donor and Recipient Size Match. J Heart Lung Transplant (2019) 38(10):1042–55. 10.1016/j.healun.2019.08.001
23.
GrimmJCValeroV3rdMagruderJTKilicADunganSPSilhanLLet alA Novel Risk Score that Incorporates Recipient and Donor Variables to Predict 1-year Mortality in the Current Era of Lung Transplantation. J Heart Lung Transplant (2015) 34(11):1449–54. 10.1016/j.healun.2015.07.001
24.
NashEFVollingCGutierrezCATullisECoonarAMcRaeKet alOutcomes of Patients with Cystic Fibrosis Undergoing Lung Transplantation with and without Cystic Fibrosis-Associated Liver Cirrhosis*. Clin Transplant (2012) 26(1):34–41. 10.1111/j.1399-0012.2010.01395.x
25.
GulackBCMezaJMLinSSHartwigMGDavisRD. Reflux and Allograft Dysfunction: Is There a Connection?Thorac Surg Clin (2015) 25(1):97–105. 10.1016/j.thorsurg.2014.09.006
26.
PosnerSZhengJWoodRKShimpiRAHartwigMGChowSCet alGastroesophageal Reflux Symptoms Are Not Sufficient to Guide Esophageal Function Testing in Lung Transplant Candidates. Dis Esophagus (2018) 31(5). 10.1093/dote/dox157
27.
SchuurmansMMBendenCInciI. Practical Approach to Early Postoperative Management of Lung Transplant Recipients. Swiss Med Wkly (2013) 143:w13773. 10.4414/smw.2013.13773
28.
LipsonDABerlinJAPalevskyHIKotloffRMTinoGBavariaJet alGiant Gastric Ulcers and Risk Factors for Gastroduodenal Mucosal Disease in Orthotopic Lung Transplant Patients. Dig Dis Sci (1998) 43(6):1177–85. 10.1023/a:1018835219474
29.
Zevallos-VillegasAAlonso-MoralejoRCambraFHermida-AnchueloAPérez-GonzálezVGámez-GarcíaPet alMorbidity and Mortality of Serious Gastrointestinal Complications after Lung Transplantation. J Cardiothorac Surg (2019) 14(1):181. 10.1186/s13019-019-0983-y
30.
HwangSSCannomRRAbbasMAEtzioniD. Diverticulitis in Transplant Patients and Patients on Chronic Corticosteroid Therapy: a Systematic Review. Dis colon rectum (2010) 53(12):1699–707. 10.1007/dcr.0b013e3181f5643c
31.
VetterDSchuurmansMMBendenCClavienP-ANocitoA. Long-term Follow-Up of Lung Transplant Recipients Supports Non-operative Treatment of Uncomplicated Diverticulitis. Clin Transpl (2016) 30(10):1264–70. 10.1111/ctr.12817
32.
ChaikriangkraiKJyothulaSJhunHYEstepJLoebeMScheininSet alImpact of Pre-operative Coronary Artery Disease on Cardiovascular Events Following Lung Transplantation. J Heart Lung Transplant (2016) 35(1):115–21. 10.1016/j.healun.2015.08.009
33.
HalloranKHirjiALiDJacksonKKapasiAMeyerSet alCoronary Artery Disease and Coronary Artery Bypass Grafting at the Time of Lung Transplantation Do Not Impact Overall Survival. Transplantation (2019) 103(10):2190–5. 10.1097/tp.0000000000002609
34.
Silva EncisoJKatoTSJinZChungCYangJTakayamaHet alEffect of Peripheral Vascular Disease on Mortality in Cardiac Transplant Recipients (From the United Network of Organ Sharing Database). Am J Cardiol (2014) 114(7):1111–5. 10.1016/j.amjcard.2014.07.027
35.
BrarAJindalRMElsterEATedlaFJohnDSumraniNet alEffect of Peripheral Vascular Disease on Kidney Allograft Outcomes. Transplantation (2013) 95(6):810–5. 10.1097/tp.0b013e31827eef36
36.
SungRSAlthoenMHowellTAMerionRM. Peripheral Vascular Occlusive Disease in Renal Transplant Recipients: Risk Factors and Impact on Kidney Allograft Survival. Transplantation (2000) 70(7):1049–54. 10.1097/00007890-200010150-00010
37.
JawitzOKRamanVBrynerBSKlapperJHartwigMG. Center Volume and Primary Graft Dysfunction in Patients Undergoing Lung Transplantation in the United States - a Cohort Study. Transpl Int (2021) 34(1):194–203. 10.1111/tri.13784
38.
LiuYLiuYSuLJiangS-j. Recipient-related Clinical Risk Factors for Primary Graft Dysfunction after Lung Transplantation: a Systematic Review and Meta-Analysis. PloS one (2014) 9(3):e92773. 10.1371/journal.pone.0092773
39.
SatoSObaTOhtaHTsukaharaYKidaGTsumiyamaEet alLevetiracetam-induced Interstitial Lung Disease in a Patient with Advanced Lung Cancer. Respir Med Case Rep (2020) 31:101241. 10.1016/j.rmcr.2020.101241
40.
HofmannPKohlerMBendenCSchuurmansMM. Tobacco Use after Lung Transplantation: A Retrospective Analysis of Patient Characteristics, Smoking Cessation Interventions, and Cessation Success Rates. Transplantation (2019) 103(6):1260–6. 10.1097/tp.0000000000002576
41.
BharatAKuoEStewardNAloushAHachemRTrulockEPet alImmunological Link between Primary Graft Dysfunction and Chronic Lung Allograft Rejection. Ann Thorac Surg (2008) 86(1):189–97. 10.1016/j.athoracsur.2008.03.073
42.
ChanEGBiancoV3rdRichardsTHayangaJWAMorrellMShigemuraNet alThe Ripple Effect of a Complication in Lung Transplantation: Evidence for Increased Long-Term Survival Risk. J Thorac Cardiovasc Surg (2016) 151(4):1171–80. 10.1016/j.jtcvs.2015.11.058
Summary
Keywords
lung transplantation, primary graft dysfunction, recipient selection, comorbidities, Charlson-Deyo-Index, chronic allograft dysfunction
Citation
Ehrsam JP, Schuurmans MM, Laager M, Opitz I and Inci I (2022) Recipient Comorbidities for Prediction of Primary Graft Dysfunction, Chronic Allograft Dysfunction and Survival After Lung Transplantation. Transpl Int 35:10451. doi: 10.3389/ti.2022.10451
Received
22 February 2022
Accepted
13 June 2022
Published
29 June 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Ehrsam, Schuurmans, Laager, Opitz and Inci.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilhan Inci, ilhan.inci@usz.ch
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.