Abstract
Prolonged warm ischemia time (WIT) has a negative prognostic value in liver transplantation (LT) using grafts procured after circulatory death (DCD). To assess the value of abdominal normothermic regional perfusion (A-NRP) associated with dual hypothermic oxygenated machine perfusion (D-HOPE) in controlled DCD LT, prospectively collected data on LTs performed between January 2016 and July 2021 were analyzed. Outcome of controlled DCD LTs performed using A-NRP + D-HOPE (n = 20) were compared to those performed with grafts procured after brain death (DBD) (n = 40), selected using propensity-score matching. DCD utilization rate was 59.5%. In the DCD group, median functional WIT, A-NRP and D-HOPE time was 43, 246, and 205 min, respectively. Early outcomes of DCD grafts recipients were comparable to those of matched DBD LTs. In DCD and DBD group, incidence of anastomotic biliary complications and ischemic cholangiopathy was 15% versus 22% (p = 0.73) and 5% versus 2% (p = 1), respectively. One-year patient and graft survival was 100% versus 95% (p = 0.18) and 90% versus 95% (p = 0.82). In conclusion, the association of A-NRP + D-HOPE in DCD LT with prolonged WIT allows achieving comparable outcomes to DBD LT.
Introduction
In liver transplantation (LT) using grafts from donors whose death has been determined by circulatory criteria (DCD), warm ischemia time (WIT) has a major impact on the outcome. Prolonged WIT has consistently been associated with an increased risk of primary non-function, ischemic cholangiopathy (IC) and inferior graft survival (1–5). In contrast with most countries with active DCD transplant programs, Italian law requires a 20-min period of absent cardiac electrical activity for death declaration (6), which significantly increases the risks associated with the use of these grafts and has slowed down implementation of DCD LT in Italy (7).
However, mainly prompted by the favourable Spanish experience with the use of abdominal normothermic regional perfusion (A-NRP) to recover DCD liver grafts from Maastricht category 2 donors (8), DCD LT was introduced in Italy in 2015 (9, 10). Given the unique characteristics of the Italian setting, use of A-NRP has been established as mandatory, while subsequent ex-situ machine perfusion (MP) has been encouraged and adopted by most centres.
A growing body of literature supports the benefits of A-NRP for livers procured from Maastricht category 3 (controlled) DCD donors (11–17). In the same setting, use of end-ischemic (dual)-hypothermic oxygenated machine perfusion (HOPE/D-HOPE) has been consistently associated with better liver graft function and lower incidence of IC as compared to static cold storage (SCS) (18–21). However, these studies reported shorter WIT compared to what can possibly be achieved in Italy.
In the Italian setting, previous studies have shown that the association of A-NRP with ex-situ machine perfusion for controlled DCD liver grafts allows achieving good LT outcomes (22–24), which appear to be comparable to those of DCD livers preserved by ultra-rapid recovery and preserved by SCS (25). However, a formal comparison with LT using livers from donors after neurologic determination of death (DBD) accounting for potential confounders and demonstrating comparable outcomes is still lacking.
Thus, the aim of the study was to report our results with the use of A-NRP + D-HOPE for controlled DCD liver grafts with prolonged WIT. To assess the effectiveness of this approach, outcomes of DCD grafts recipients were compared to those of a matched cohort of DBD LTs, selected using propensity score matching.
Materials and Methods
Prospectively collected data on adult (≥18-year-old) patients who underwent LT at our centre from January 2016 to July 2021 were retrospectively analyzed. Collected data included donor and recipient baseline characteristics, operational details, and prognostic scores (26, 27). The UK-DCD risk score (4), a prognostic score for DCD LT based on 4 donor and 3 recipient variables, was used to grade the risk profile associated with each case. Recipients of a combined transplant, retransplant, partial graft or suffering from on-table death were excluded. To limit confounding, recipients of a DBD graft treated with any type of machine perfusion were also excluded, as well as recipients of Maastricht category 2 DCD grafts and of Maastricht category 3 DCD grafts treated with a machine perfusion modality other than D-HOPE. To control selection bias, two comparable cohorts of DBD and controlled DCD LTs were selected using 1:2 propensity score matching. Minimal patient follow-up was 6 months. The study was conducted according to the principles of the Istanbul and Helsinki declarations and was approved by the ethics committee of our Institution (protocol 506/2021).
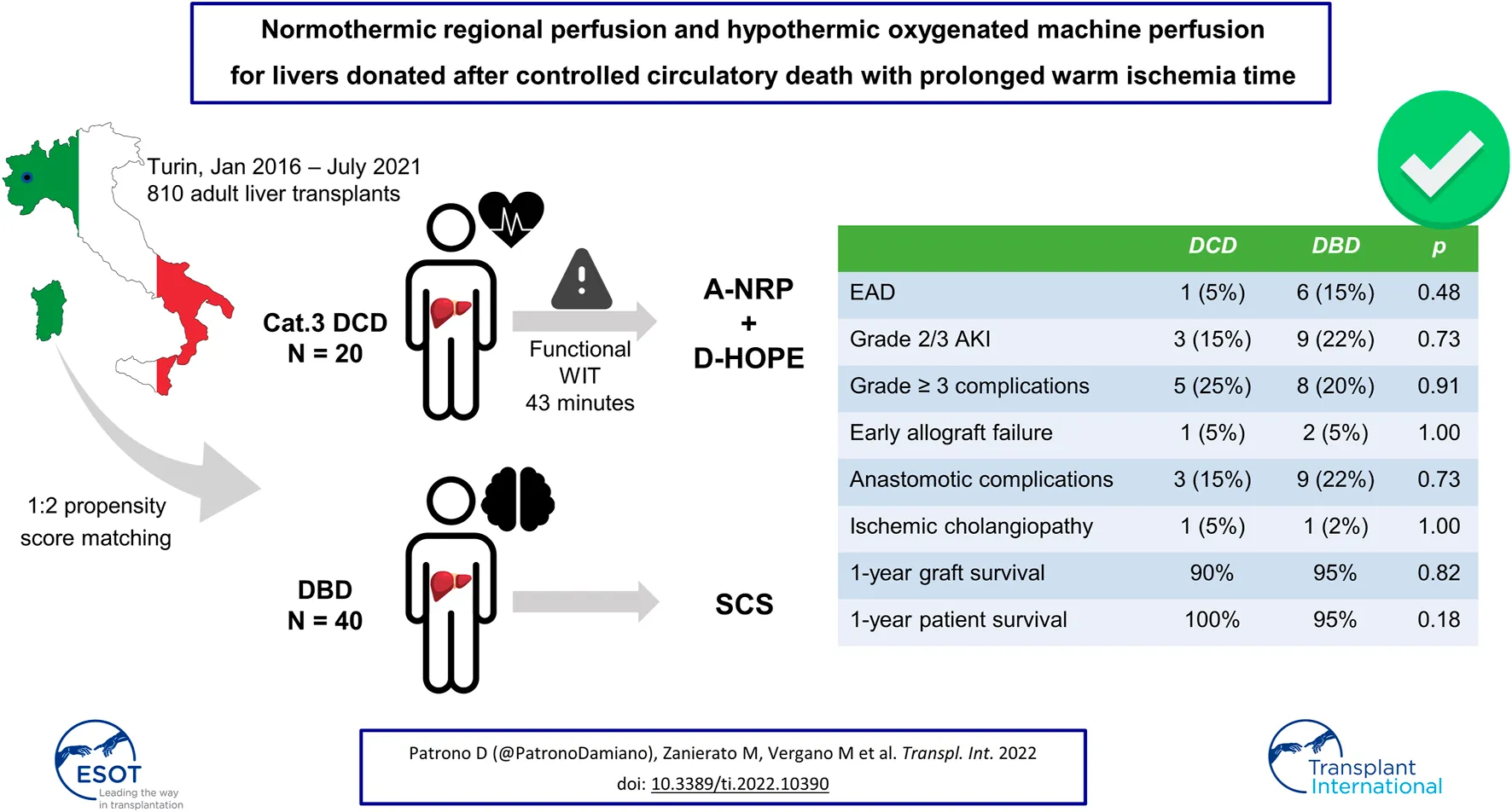
Our procurement and machine perfusion protocols are depicted in Figure 1. Briefly, withdrawal of life-sustaining treatment (WLST) took place in the operating theatre, after guidewires for subsequent femoral vessels cannulation had been placed under ultrasound guidance (pre-mortem cannulation is not allowed in Italy). At the onset of functional warm ischemia (peripheral O2 saturation ≤70% or systolic blood pressure ≤50 mmHg, whichever occurred first) 300 IU/kg heparin was administered. After 20-min electrical asystole, death was declared, femoral vessels were cannulated and descending aorta was occluded by an endovascular balloon or a surgical clamp, depending on theatre logistic, after which A-NRP was started. During A-NRP, pump flow was maintained ≥1.7 L/min/m2 and temperature at 35–36°C (28). Target perfusion pressure was 55–70 mmHg, which was sustained using low dose vasopressin or norepinephrine when necessary, in addition to flow settings and fluid replacement. The circuit sweep gas levels (FiO2 and air flow) were adjusted to maintain PaCO2 between 35 and 45 mmHg, SaO2 about 96–98%, and SvO2 > 60%. If needed, packed red blood cells were transfused to maintain haematocrit ≥20%. Heparin boluses were administered based on activated clotting time values. Blood samples were obtained prior to A-NRP start, at 30 min and then hourly to adjust A-NRP parameters (gas flow, blood flow, FiO2, pump speed) and to assess liver injury and function. Target A-NRP duration was 4 h and it was never less than 2 h or more than 6 h. During A-NRP, liver viability assessment was based on a modified version of the criteria proposed by De Carlis et al. (29), including pump flow >1.7 lt/min/m2, transaminase level <1,000 IU/L, downward lactate trend, absence of significant (≥15%) macrovesicular steatosis or Ishak >1 fibrosis, good liver and abdominal viscera perfusion, and evidence of bile production. A liver biopsy was systematically obtained to rule out significant necrosis or macrovesicular steatosis. At the end of A-NRP, the liver graft was cold flushed with Celsior (IGL, Lissieu, France) solution through the arterial cannula and trough a portal vein tributary. Liver was prepared on the backtable immediately upon arrival at our transplant centre and subsequently underwent a minimum of 2 h of D-HOPE using the LiverAssist device (XVivo, Groningen, Netherlands), primed with 3 lt of Belzer MP solution (BridgeToLife, Northbrook, IL). Temperature, portal vein and hepatic artery pressure were set at 8–10°C, 3–5 mmHg and 25 mmHg, respectively. D-HOPE was not used with the purpose of viability assessment and all grafts treated by D-HOPE were subsequently transplanted. At the end of recipient hepatectomy, the liver was disconnected from the device and brought to the operating table for implantation.
FIGURE 1
DCD procurement protocol. Abbreviations: WLST, withdrawal of life-sustaining treatment; SBP, systemic blood pressure; A-NRP, abdominal normothermic regional perfusion; D-HOPE, dual hypothermic oxygenated machine perfusion; WIT, warm ischemia time; IVC, inferior vena cava.
In DBD group, the liver was flushed with Celsior and preserved by static cold storage until implantation into the recipient. In both groups, the liver was flushed with chilled 5% albumin solution before implantation.
As a rule, liver transplant was performed by the piggyback technique with portal reperfusion first. Following hepatic artery anastomosis, an end-to-end biliary anastomosis was performed using a 2.5 mm T-tube. In all patients graft histology was assessed on time-0 biopsies, which were systematically obtained at the end of transplant operation. Standard immunosuppression included basiliximab, tacrolimus, steroids and mycophenolate mofetil, and was not modified according to treatment group.
Early outcome endpoints included rate of post-reperfusion syndrome (30, 31), transaminase peak, early allograft dysfunction (32), rate and severity of acute kidney injury (AKI) (33), requirement for renal replacement therapy, hospital and intensive care unit (ICU) stay, postoperative complications (34, 35), and the rate of early graft failure (EAF), defined as death of relisting for LT withing 90 days from transplant.
Post-reperfusion syndrome was defined as a drop in mean arterial pression ≥30% from the baseline for at least 1 min and within 5 min from reperfusion (30), whereas severe post-reperfusion syndrome was defined as the onset of severe hemodynamic instability, persistent hypotension, cardiac arrest or hemodynamically significant arrhythmias (31). EAD and AKI were defined according to Olthoff et al. (32) and KDIGO guidelines (33). Postoperative complications were graded according to Clavien-Dindo classification (34), which was also used to calculate comprehensive complication index (CCI) (35).
Biliary complications (36) were diagnosed based on the 3-month cholangiogram obtained before removing the T-tube, or by magnetic resonance cholangiopancreatography (MRCP), which was performed if clinically indicated. Recipients of a DCD graft underwent systematic 6-month and 12-month MRCP.
Variables are presented as number (percentage) of median (interquartile range), as appropriate, and compared using Fisher’s, Chi-square and Mann-Whitney tests. To control selection bias, 1:2 propensity score matching without replacement and using the nearest method was used to select two patient cohorts with comparable characteristics. Variables included in the model were recipient age, body mass index (BMI) and model for end-stage liver disease (MELD) score, hepatocellular carcinoma (HCC) as an indication for LT, donor age and BMI, percentage of macrovesicular steatosis and presence of macrovesicular steatosis ≥15%. Standardized mean differences were used to assess balance obtained by propensity score matching. Patient and graft survival was analyzed using Kaplan-Meier curves. Statistical analysis was performed using R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria).
Results
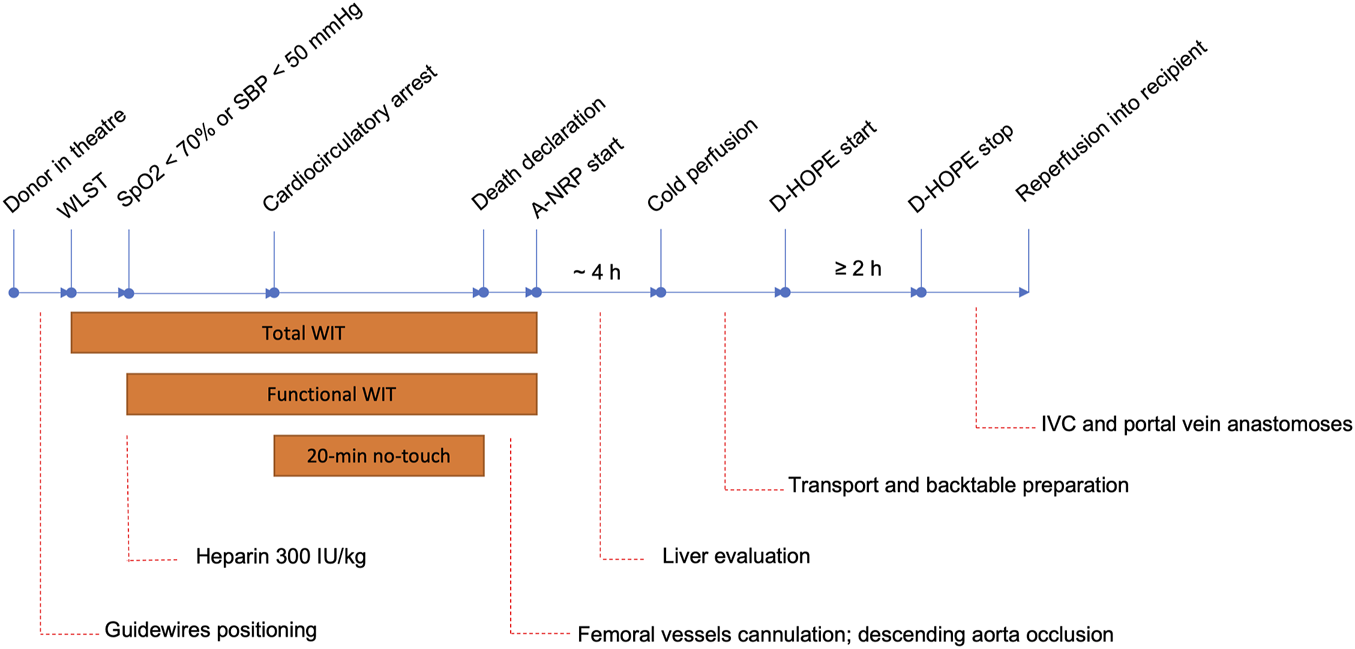
During study period, 810 adult LTs were performed, of which 26 using organs proceeding from a DCD donor (cat. 3, n = 22; cat. 2, n = 4). A total of 37 category 3 DCD donors were signalled in our region during study period, of which 22 were transplanted by our centre. As per Italian regulations, livers from regional DCD donors were allocated locally to our centre, which is the only liver transplant centre in our region, and referred elsewhere only upon refusal by our unit. Four livers were refused based on donor characteristics and the organs were reallocated to other centres. Three of these grafts were successfully transplanted, whereas one was discarded during A-NRP due to elevated transaminases and lack of lactate clearance. Of the remaining 11 livers, 6 were discarded by our and all other Italian centres based on donor features, whereas of 5 offers initially accepted by our centre, 2 were subsequently discarded due to excessive functional WIT, and 3 during A-NRP. The reason to discard the liver during A-NRP was mainly elevated transaminases, which was associated to persistently elevated lactate levels in one case and evidence of gallbladder and bile duct necrosis in another. No liver was discarded based on histological findings. Overall utilization rate of livers from category 3 DCD donors was 25/37 (67.6%), whereas it was 22/37 (59.5%) if we consider only those transplanted at our centre.
Based on exclusion criteria, 229 and 6 patients were excluded from DBD and DCD group, respectively (Figure 2). In the DCD group, besides 4 recipients of livers from category 2 DCD donors, 2 further cases, including one retransplant, were excluded due to the use of normothermic machine perfusion instead of D-HOPE. Thus, 555 DBD and 20 DCD liver transplants were included for analysis. Finally, outcomes of the 20 DCD LTs were compared to those of 40 recipients of a DBD graft, selected by 1:2 propensity score matching.
FIGURE 2
Patient selection flowchart. *some patients met more than one exclusion criterium. NMP, normothermic machine perfusion.
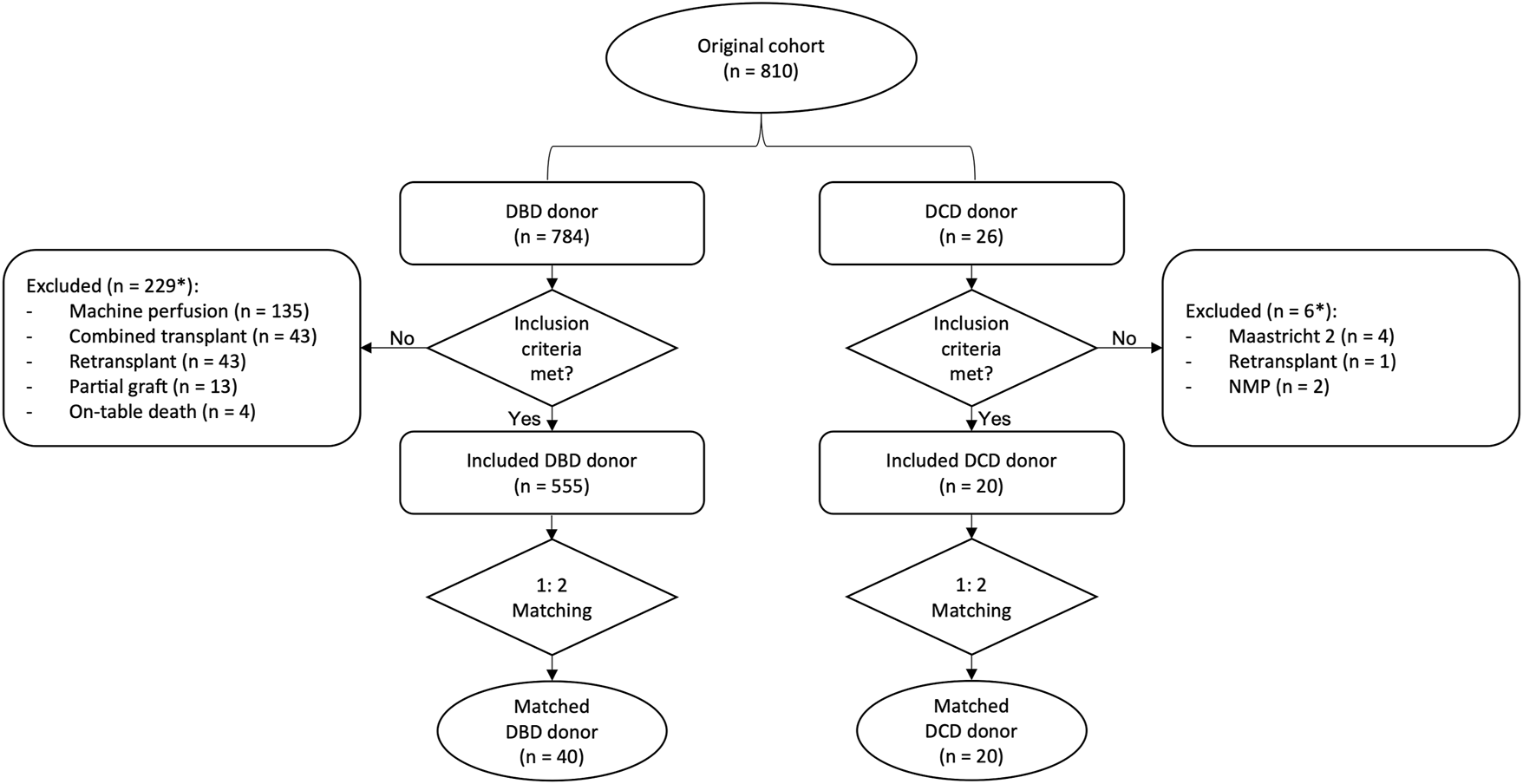
Baseline patient and donor characteristics and operational details are summarized in Table 1. In the DCD group, median donor age and BMI were 60.1 (55.1, 61.5) and 25.0 [23.0, 26.1], and only one liver had 15% macrovesicular steatosis, reflecting our policy of avoiding overlap of additional donor risk factors in this high-risk cohort, characterized by a functional WIT of 43 (35, 46) min. A-NRP and D-HOPE times were 246 (221, 269) and 205 (146, 277) min, respectively. DCD livers were preferentially allocated to low-MELD (10.5 [8.8, 14.5]) patients, with HCC being the indication for LT in 80% of cases. However, with increasing experience, livers from elderly donors were also accepted and procured organs were more frequently allocated to higher-MELD recipients (Figure 3). Despite donor and recipient selection, median UK-DCD risk score (4) was 13 (11, 14) with 17 cases being classified as “futile” and 3 as “high-risk”.
TABLE 1
| Whole cohort | Matched cohort | ||||||
|---|---|---|---|---|---|---|---|
| DBD | DCD | p | SMD | DBD | DCD | SMD | |
| n | 555 | 20 | 40 | 20 | |||
| Rec. age | 57.5 [52.4, 62.1] | 60.7 [57.4, 66.7] | 0.02 | 0.64 | 60.6 [56.2, 65.6] | 60.7 [57.4, 66.7] | 0.04 |
| Gender (male) | 404 (73) | 16 (80) | 0.65 | 0.17 | 30 (75) | 16 (80) | 0.12 |
| Rec. BMI | 25.0 [22.7, 27.7] | 25.3 [22.6, 27.3] | 0.90 | 0.05 | 25.2 [22.5, 27.8] | 25.3 [22.6, 27.3] | 0.01 |
| Indication | 0.28 | 0.65 | 0.76 | ||||
| Viral hepatitis | 276 (50) | 9 (45) | 27 (68) | 9 (45) | |||
| Alcoholic cirrhosis | 98 (18) | 6 (30) | 7 (18) | 6 (30) | |||
| Cholestatic disease | 39 (7) | 2 (10) | 0 (0) | 2 (10) | |||
| NASH | 17 (3) | 2 (10) | 1 (2) | 2 (10) | |||
| Autoimmune | 16 (3) | 0 (0) | 0 (0) | 0 (0) | |||
| Acute liver failure | 3 (1) | 0 (0) | 0 (0) | 0 (0) | |||
| Other | 106 (19) | 1 (5) | 5 (12) | 1 (5) | |||
| MELD | 13.0 [9.0, 18.0] | 10.5 [8.8, 14.5] | 0.17 | 0.21 | 11.5 [8.0, 17.2] | 10.5 [8.8, 14.5] | 0.10 |
| Creatinine (mg/dl) | 0.8 [0.7, 1.1] | 0.8 [0.7, 1.0] | 0.95 | 0.02 | 0.9 [0.7, 1.2] | 0.8 [0.7, 1.0] | 0.20 |
| Dialysis pre-LT | 11 (2) | 0 (0) | 1.00 | 0.20 | 1 (2) | 0 (0) | 0.23 |
| Prev. abdo. surgery | 206 (37) | 10 (50) | 0.35 | 0.26 | 21 (52) | 10 (50) | 0.05 |
| Life support | 17 (3) | 1 (5) | 1.00 | 0.10 | 1 (2) | 1 (5) | 0.13 |
| Ascites | 211 (38) | 7 (35) | 0.96 | 0.06 | 14 (35) | 7 (35) | <0.01 |
| Encephalopathy | 114 (21) | 2 (10) | 0.38 | 0.30 | 7 (18) | 2 (10) | 0.22 |
| HCC | 296 (53) | 16 (80) | 0.03 | 0.59 | 33 (82) | 16 (80) | 0.06 |
| Donor age | 65.4 [52.4, 74.4] | 60.1 [55.1, 61.5] | 0.13 | 0.30 | 63.1 [44.8, 71.7] | 60.1 [55.1, 61.5] | 0.04 |
| Donor BMI | 25.3 [22.9, 27.7] | 25.0 [23.0, 26.1] | 0.57 | 0.17 | 25.3 [23.3, 27.6] | 25.0 [23.0, 26.1] | 0.14 |
| Macrosteatosis (%) | 1.0 [0.0, 5.0] | 0.0 [0.0, 1.2] | 0.05 | 0.35 | 0.0 [0.0, 3.5] | 0.0 [0.0, 1.2] | 0.02 |
| Macrosteatosis ≥15% | 64 (12) | 1 (5) | 0.57 | 0.24 | 2 (5) | 1 (5) | <0.01 |
| Microsteatosis (%) | 10.0 [1.0, 25.0] | 5.0 [0.0, 10.0] | 0.04 | 0.53 | 10.0 [4.5, 20.0] | 5.0 [0.0, 10.0] | 0.36 |
| D-MELD | 800 [573, 1117] | 542 [488, 1014] | 0.05 | 0.33 | 699 [533, 977] | 542 [488, 1014] | 0.12 |
| BAR | 5.0 [3.0, 19.0] | 5.0 [3.0, 8.0] | 0.99 | 0.18 | 5.0 [3.0, 17.0] | 5.0 [3.0, 8.0] | 0.09 |
| WIT (min) | 43 [40, 48] | 43 [40, 48] | |||||
| Functional WIT (min) | 43 [35, 46] | 43 [35, 46] | |||||
| A-NRP time (min) | 246 [221, 269] | 246 [221, 269] | |||||
| CIT (min) | 431 [379, 482] | 261 [229, 295] | <0.01 | 2.06 | 418 [375, 510] | 261 [229, 295] | 1.86 |
| D-HOPE time (min) | 205 [146, 277] | 205 [146, 277] | |||||
| Total pres. time (min) | 431 [379, 482] | 492 [426, 531] | 0.01 | 0.65 | 418 [375, 510] | 492 [426, 531] | 0.58 |
| Portal rep. time (min) | 23.0 [21.0, 27.0] | 22.0 [20.5, 26.2] | 0.47 | 0.19 | 23.0 [21.0, 26.2] | 22.0 [20.5, 26.2] | 0.01 |
| Total rep. time (min) | 38.0 [24.0, 50.2] | 48.5 [42.0, 59.5] | 0.01 | 0.51 | 41.0 [24.0, 55.2] | 48.5 [42.0, 59.5] | 0.41 |
| PRBC units (n) | 3.0 [0.0, 8.0] | 2.5 [0.0, 7.2] | 0.70 | 0.04 | 5.0 [0.8, 9.2] | 2.5 [0.0, 7.2] | 0.01 |
| Graft weight (gr) | 1490 [1290, 1720] | 1455 [1222, 1610] | 0.39 | 0.19 | 1475 [1295, 1692] | 1455 [1222, 1610] | 0.09 |
Baseline covariates balance.
Abbreviations: SMD, standardized mean difference; BMI, body mass index; NASH, non-alcoholic steatohepatitis; MELD, model for end-stage liver disease; prev, previous; HCC, hepatocellular carcinoma; D-MELD, donor age * MELD score; BAR, balance of risk score; WIT, warm ischemia time; A-NRP, abdominal normothermic regional perfusion; CIT, cold ischemia time; D-HOPE, dual hypothermic oxygenated machine perfusion; pres, preservation; rep, reperfusion; PRBC, packed red blood cells.
FIGURE 3
Scatter plot depicting donor age and recipient MELD as a function of study period. During study period, donors of increasing age were considered, and DCD grafts were more frequently allocated to higher-MELD recipients (arrows).
Patient cohorts selected by propensity score matching showed good comparability, as reflected by a standardized mean difference ≤0.10 for all major confounders, including recipient age, BMI and MELD score, HCC as the indication for LT, donor age, graft macrovesicular steatosis, balance of risk (BAR) score and portal reperfusion time (Table 1).
Outcomes in the unmatched and matched cohort are reported in Table 2. Overall, early outcomes in the DCD group were comparable to those observed in the DBD group.
TABLE 2
| Whole cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|
| DBD | DCD | p | DBD | DCD | p | |
| n | 555 | 20 | 40 | 20 | ||
| Severe PRS | 77 (14) | 3 (15) | 1.00 | 4 (10) | 3 (15) | 0.89 |
| End-LT lactate (mmol/l) | 2.0 [1.4, 2.8] | 1.6 [1.0, 2.4] | 0.13 | 2.0 [1.4, 2.9] | 1.6 [1.0, 2.4] | 0.26 |
| AST peak (IU/L) | 1111 [692, 1752] | 761 [589, 1345] | 0.13 | 937 [663, 1438] | 761 [589, 1345] | 0.63 |
| ALT peak (IU/L) | 702 [448, 1126] | 461 [385, 608] | 0.01 | 632 [360, 835] | 461 [385, 608] | 0.18 |
| EAD | 157 (28) | 1 (5) | 0.04 | 6 (15) | 1 (5) | 0.48 |
| AKI stage | 0.53 | 0.27 | ||||
| 0 | 178 (32) | 8 (40) | 10 (25) | 8 (40) | ||
| 1 | 226 (41) | 9 (45) | 21 (52) | 9 (45) | ||
| 2 | 107 (19) | 3 (15) | 4 (10) | 3 (15) | ||
| 3 | 44 (8) | 0 (0) | 5 (12) | 0 (0) | ||
| Grade 2/3 AKI | 151 (27) | 3 (15) | 0.34 | 9 (22) | 3 (15) | 0.73 |
| Renal replacement therapy | 13 (2) | 0 (0) | 1.00 | 0 (0) | 0 (0) | NA |
| Early rejection | 46 (8) | 1 (5) | 0.91 | 3 (8) | 1 (5) | 1.00 |
| Grade ≥3 complications | 126 (23) | 5 (25) | 1.00 | 8 (20) | 5 (25) | 0.91 |
| ICU stay (days) | 3.0 [2.0, 5.0] | 4.0 [2.0, 5.0] | 0.92 | 4.0 [2.0, 6.0] | 4.0 [2.0, 5.0] | 0.55 |
| Hospital stay (days) | 12.0 [9.0, 17.0] | 10.0 [8.0, 19.5] | 0.59 | 12.0 [9.0, 19.0] | 10.0 [8.0, 19.5] | 0.35 |
| Hospital CCI | 22.6 [12.0, 33.7] | 16.5 [0.0, 33.9] | 0.10 | 21.8 [8.7, 35.4] | 16.5 [0.0, 33.9] | 0.26 |
| Early allograft failure | 28 (5) | 1 (5) | 1.00 | 2 (5) | 1 (5) | 1.00 |
| Biliary complications | ||||||
| Anastomotic | 85 (15) | 3 (15) | 1.00 | 9 (22) | 3 (15) | 0.73 |
| Fistula | 10 (2) | 1 (5) | 0.85 | 2 (5) | 1 (5) | 1.00 |
| Stricture | 75 (14) | 2 (10) | 0.91 | 7 (18) | 2 (10) | 0.70 |
| IC | 28 (5) | 1 (5) | 1.00 | 1 (2) | 1 (5) | 1.00 |
| Treatment | 0.06 | 0.15 | ||||
| Operational | 69 (71) | 1 (33) | 7 (78) | 1 (33) | ||
| Surgery | 24 (25) | 1 (33) | 2 (22) | 1 (33) | ||
| Retransplant | 4 (4) | 1 (33) | 0 (0) | 1 (33) | ||
| N° of treatments | 2.0 [1.0, 3.0] | 3.0 [2.5, 4.5] | 0.33 | 2.0 [2.0, 3.0] | 3.0 [2.5, 4.5] | 0.43 |
| Determining graft loss | 5 (1) | 1 (5) | 0.51 | 0 (0) | 1 (5) | 0.72 |
| Determining patient death | 1 (0) | 0 (0) | 1.00 | 0 (0) | 0 (0) | NA |
Outcome.
Abbreviations: PRS, post-reperfusion syndrome; LT, liver transplant; EAD, early allograft dysfunction; AKI, acute kidney injury; ICU, intensive care unit; CCI, comprehensive complication index; IC, ischemic cholangiopathy.
In the DCD and DBD group, EAD and grade 2/3 AKI rates were 5% versus 15% and 15% versus 22%, respectively, with no patient requiring renal replacement therapy after LT. Five (25%) and 8 (20%) recipients of a DCD or DBD liver, respectively, developed grade ≥3 complications and median comprehensive complication index was 16.5 (0.0, 33.9) versus 21.8 (8.7, 35.4). Intensive care unit and hospital length of stay was 4 (2, 5) versus 4 (2, 6) and 10 (8, 19.5) versus 12 (9, 19) days, respectively. Two grafts were lost in the DCD group, which were the first and the second in our series. The first graft loss resulted from a hepatic artery injury that occurred during an attempt at performing hepaticojejunostomy for a late biliary fistula 97 days after LT. The vascular injury resulted from the severe inflammatory reaction caused by the biloma involving the porta hepatis and was deemed not amenable to repair. The second graft loss was caused by hepatic artery thrombosis occurring on postoperative day 2. Despite the graft was showing good function, large necrotic areas were apparent at computed tomography scan, so a decision was made to relist the recipient for urgent retransplantation. Both patients were successfully retransplanted.
The rate of anastomotic biliary complications and ischemic cholangiopathy was comparable between groups (Table 2). In particular, only one case of IC was observed in the DCD group. This patient had a percutaneous biliary drain inserted before undergoing hepaticojejunostomy for a tight anastomotic stricture. Cholangiogram showed an isolated posterior duct stricture, likely representing an incidental finding. Patient was treated with a single balloon dilatation and has neither clinical nor radiological evidence of recurrence 8 months after the procedure.
Median follow-up was 40 (21, 56) and 15.5 (12, 27) months in the DBD and DCD group, respectively. Graft and patient survival was comparable between groups (Figure 4). In the matched cohort, 1-year patient survival in the DCD and DBD group was 100% (confidence interval [CI] = 100%, 100%) and 95% (CI = 88.5%, 100%), respectively, whereas 1-year graft survival was 90% (CI = 77.8%, 100%) and 95% (CI = 88.5%, 100%).
FIGURE 4
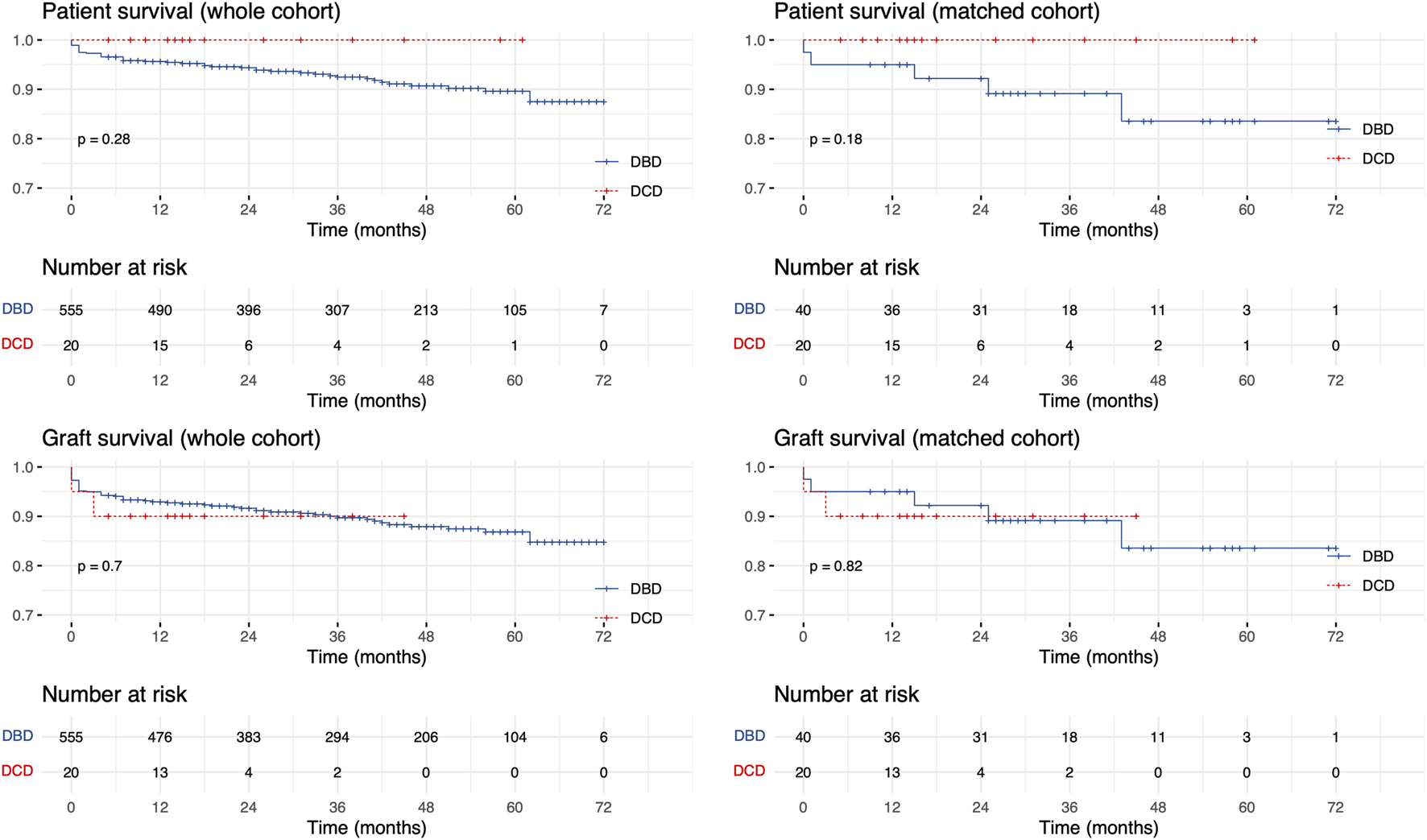
Kaplan-Meier patient and graft survival curves in the unmatched and matched cohorts.
Discussion
This study shows that a combination of A-NRP followed by D-HOPE is effective in preserving grafts from controlled DCD donors with prolonged WIT and allows obtaining comparable outcomes to DBD LT. These results appear to be even more remarkable if some peculiarities of the Italian setting are considered. Besides the 20-min no-touch time, which is unique among countries with active DCD programs (6), pre-mortem cannulation is not allowed in Italy, which further prolongs WIT due to the time necessary to cannulate femoral vessels and occlude the descending aorta (Figure 1). Furthermore, as the required 20 min of flat EKG recording are preceded by a variable time of pulseless electric activity, procured organs are exposed to a no-flow time that is frequently much longer than the 20-min no-touch time. If these livers were procured by ultra-rapid recovery and preserved by static cold storage, a poor outcome would be expected (1–4). In contrast, reconditioning and preservation by A-NRP + D-HOPE appears to allow obtaining good results, which are not different from those observed after DBD LT. It is worth noting that, despite initial concerns and logistic obstacles, our ∼60% utilization rate compares favourably with that observed in other realities (37, 38).
Overall, our results confirm the benefits of both A-NRP and D-HOPE in controlled DCD LT. As compared to ultra-rapid recovery followed by static cold storage, use of A-NRP has been associated with better graft function, lower rate of overall biliary complications and ischemic cholangiopathy, and improved graft survival (11–13, 15–17, 39). A recent large Spanish study has shown that use of A-NRP alone in DCD LT allows achieving comparable outcome to DBD LT (13). Additionally, use of A-NRP appears to positively impact on utilization rate and post-transplant function of other abdominal organs, especially kidneys (40, 41). On the other hand, DCD LT is the setting in which the advantages of end-ischemic D-HOPE have been more convincingly demonstrated (18, 19, 21, 42–44), with a recent randomized controlled trial showing that use of D-HOPE in this context is associated with a significant reduction of symptomatic non-anastomotic biliary stricture incidence from 18% to 6% (19). However, these data come from countries where local regulations allow usually limiting WIT to 10–15 min, which is much shorter than what is currently observed in Italy. Therefore, Italian centres have frequently considered to combine these two approaches. In Italy, successful use of controlled DCD donors by combining A-NRP and D-HOPE or normothermic machine perfusion has already been reported (9, 10, 22–24, 29), with a recent study by De Carlis et al.(25) showing that, despite longer WIT, outcome of liver grafts procured by this approach is comparable to those of DCD liver grafts procured by ultra-rapid recovery and SCS. To our knowledge, the present study is the first suggesting that the outcome of controlled DCD LT performed by combining A-NRP and D-HOPE, despite a functional WIT almost invariably exceeding 40 min, is not inferior to that of matched DBD LT.
Undoubtedly, these favourable results also issue from accurate donor selection and liver function assessment during A-NRP. In our experience, four (12.9%) initially accepted grafts were discarded based on parameters obtained during A-NRP. Different criteria for liver viability assessment during A-NRP have been proposed in different countries (8, 16, 17, 45, 46). Given the expected long WIT, we chose to adopt a modified version of the rather unrestrictive criteria proposed by De Carlis et al.(29). These criteria were not modified during study period and are still currently adopted at our centre. The good outcome observed in our series seems to confirm their validity. However, these data must be considered preliminary and future larger studies should investigate whether these criteria could be safely expanded further.
As LT outcomes are also influenced by recipient condition (26, 27), it is likely that recipient selection also played a role in achieving the good results observed in this series. This is the reason why, in order to allow a meaningful comparison, recipient characteristics were accounted for in the matching process. However, although initially DCD livers were preferentially allocated to low-MELD patients undergoing LT for HCC, the good results observed during the initial phases of this study fostered an increased confidence with DCD grafts utilization, which led to consider donor of progressively increasing age and to allocate DCD grafts also to patients with severe hepatic disease (Figure 3), without observing any detrimental effect on outcomes. This was also associated with an increasing number of DCD LTs per year (Figure 3). Overall, these findings are in keeping with the good outcome achieved and reflect how utilization of DCD liver grafts has become standard practice.
Limitations of our study include retrospective single-centre design and limited numerosity. Given the exploratory nature of this analysis, formal sample size calculation was not made. Also, as the majority of DCD LTs were performed in 2020–2021, follow-up was shorter in DCD group. Although 6-months minimal follow-up should have allowed capturing the majority of biliary complications, late-onset complications could have been missed. We are aware that an updated definition of functional WIT has been recently introduced (47). However, all cases included in this study were antecedent to its introduction and a retrospective recalculation of functional WIT was not possible. Finally, as all grafts included in this study were treated with D-HOPE, we could not evaluate the additional value of D-HOPE after A-NRP. It could be argued that use of machine perfusion could be omitted in selected cases, whereas additional viability assessment by normothermic machine perfusion could be indicated in others (48). In our experience, use of D-HOPE has been systematic for grafts meeting all viability criteria during A-NRP, which are those included in this series. So far, use of normothermic machine perfusion has been limited to cases characterized by doubtful evaluation during A-NRP (24), or in which logistics constraints imposed prolonging preservation time. Well designed and appropriately powered randomized studies are needed to define when and by which modality machine perfusion after A-NRP is indicated in DCD LT.
In conclusion, despite apparently prohibitive WIT, outcome of LT using livers from controlled DCD donors treated by a combination of A-NRP and D-HOPE is comparable to that of DBD LT, suggesting that a wider implementation of this approach could contribute improving the results of DCD LT and expand donor pool. Larger studies are required to confirm these findings, refine our evaluation process, and establish when and by which modality machine perfusion is indicated in this setting.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Comitato Etico Interaziendale A.O.U. Città della Salute e della Scienza di Torino–A.O. Ordine Mauriziano–A.S.L. Città di Torino. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
DP designed the study, collected and analysed data, and drafted the paper; MZ, MV, and RP contributed to data collection, data analysis and paper drafting; CM and ED collected data and critically revised the paper; GR, SC, SM, and DC contributed to data collection and critically revised the manuscript; SL, RB, and RR critically revised the manuscript.
Acknowledgments
The authors are deeply thankful to all healthcare professionals who, with astonishing dedication and despite the disruption caused by Covid-19 pandemic in recent years, have supported and keep supporting transplant activity in our region.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Blok JJ Detry O Putter H Rogiers X Porte RJ van Hoek B et al Longterm Results of Liver Transplantation from Donation after Circulatory Death. Liver Transpl (2016) 22:1107–14. 10.1002/lt.24449
2.
Coffey JC Wanis KN Monbaliu D Gilbo N Selzner M Vachharajani N et al The Influence of Functional Warm Ischemia Time on DCD Liver Transplant Recipients' Outcomes. Clin Transpl (2017) 31:31. 10.1111/ctr.13068
3.
Mateo R Cho Y Singh G Stapfer M Donovan J Kahn J et al Risk Factors for Graft Survival after Liver Transplantation from Donation after Cardiac Death Donors: an Analysis of OPTN/UNOS Data. Am J Transpl (2006) 6:791–6. 10.1111/j.1600-6143.2006.01243.x
4.
Schlegel A Kalisvaart M Scalera I Laing RW Mergental H Mirza DF et al The UK DCD Risk Score: A New Proposal to Define Futility in Donation-After-Circulatory-Death Liver Transplantation. J Hepatol (2018) 68:456–64. 10.1016/j.jhep.2017.10.034
5.
Kalisvaart M Schlegel A Umbro I de Haan JE Scalera I Polak WG et al The Impact of Combined Warm Ischemia Time on Development of Acute Kidney Injury in Donation after Circulatory Death Liver Transplantation. Transplantation (2018) 102:783–93. 10.1097/tp.0000000000002085
6.
Lomero M Gardiner D Coll E Haase‐Kromwijk B Procaccio F Immer F et al Donation after Circulatory Death Today: an Updated Overview of the European Landscape. Transpl Int (2020) 33:76–88. 10.1111/tri.13506
7.
Vergano M Magavern E Baroncelli F Frisenda V Fonsato A Artusio D et al Making a Case for Controlled Organ Donation after Cardiac Death: the story of Italy's First Experience. J Crit Care (2017) 38:129–31. 10.1016/j.jcrc.2016.10.028
8.
Fondevila C Hessheimer AJ Ruiz A Calatayud D Ferrer J Charco R et al Liver Transplant Using Donors after Unexpected Cardiac Death: Novel Preservation Protocol and Acceptance Criteria. Am J Transpl (2007) 7:1849–55. 10.1111/j.1600-6143.2007.01846.x
9.
De Carlis L De Carlis R Lauterio A Di Sandro S Ferla F Zanierato M . Sequential Use of Normothermic Regional Perfusion and Hypothermic Machine Perfusion in Donation after Cardiac Death Liver Transplantation with Extended Warm Ischemia Time. Transplantation (2016) 100:e101–e102. 10.1097/tp.0000000000001419
10.
De Carlis L Lauterio A De Carlis R Ferla F Di Sandro S . Donation after Cardiac Death Liver Transplantation after More Than 20 minutes of Circulatory Arrest and Normothermic Regional Perfusion. Transplantation (2016) 100:e21–e22. 10.1097/tp.0000000000001136
11.
Hessheimer AJ Coll E Torres F Ruíz P Gastaca M Rivas JI et al Normothermic Regional Perfusion vs. Super-rapid Recovery in Controlled Donation after Circulatory Death Liver Transplantation. J Hepatol (2019) 70:658–65. 10.1016/j.jhep.2018.12.013
12.
Ruiz P Gastaca M Bustamante FJ Ventoso A Palomares I Prieto M et al Favorable Outcomes after Liver Transplantation with Normothermic Regional Perfusion from Donors after Circulatory Death: A Single-center Experience. Transplantation (2019) 103:938–43. 10.1097/tp.0000000000002391
13.
Ruiz P Valdivieso A Palomares I Prieto M Ventoso A Salvador P et al Similar Results in Liver Transplantation from Controlled Donation after Circulatory Death Donors with Normothermic Regional Perfusion and Donation after Brain Death Donors: A Case‐Matched Single‐Center Study. Liver Transpl (2021) 27:1747–57. 10.1002/lt.26281
14.
Savier E Lim C Rayar M Orlando F Boudjema K Mohkam K et al Favorable Outcomes of Liver Transplantation from Controlled Circulatory Death Donors Using Normothermic Regional Perfusion Compared to Brain Death Donors. Transplantation (2020) 104:1943–51. 10.1097/tp.0000000000003372
15.
Oniscu GC Randle LV Muiesan P Butler AJ Currie IS Perera MTPR et al In SituNormothermic Regional Perfusion for Controlled Donation after Circulatory Death-The United Kingdom Experience. Am J Transpl (2014) 14:2846–54. 10.1111/ajt.12927
16.
Watson CJE Hunt F Messer S Currie I Large S Sutherland A et al In Situ normothermic Perfusion of Livers in Controlled Circulatory Death Donation May Prevent Ischemic Cholangiopathy and Improve Graft Survival. Am J Transpl (2019) 19:1745–58. 10.1111/ajt.15241
17.
Hessheimer AJ de la Rosa G Gastaca M Ruíz P Otero A Gómez M et al Abdominal Normothermic Regional Perfusion in Controlled Donation after Circulatory Determination of Death Liver Transplantation: Outcomes and Risk Factors for Graft Loss. Am J Transpl (2021) 22:1169–81. 10.1111/ajt.16899
18.
van Rijn R Karimian N Matton APM Burlage LC Westerkamp AC van den Berg AP et al Dual Hypothermic Oxygenated Machine Perfusion in Liver Transplants Donated after Circulatory Death. Br J Surg (2017) 104:907–17. 10.1002/bjs.10515
19.
van Rijn R Schurink IJ de Vries Y van den Berg AP Cortes Cerisuelo M Darwish Murad S et al Hypothermic Machine Perfusion in Liver Transplantation - A Randomized Trial. N Engl J Med (2021) 384:1391–401. 10.1056/nejmoa2031532
20.
van Rijn R van Leeuwen OB Matton APM Burlage LC Wiersema-Buist J van den Heuvel MC et al Hypothermic Oxygenated Machine Perfusion Reduces Bile Duct Reperfusion Injury after Transplantation of Donation after Circulatory Death Livers. Liver Transpl (2018) 24:655–64. 10.1002/lt.25023
21.
Schlegel A Muller X Kalisvaart M Muellhaupt B Perera MTPR Isaac JR et al Outcomes of DCD Liver Transplantation Using Organs Treated by Hypothermic Oxygenated Perfusion before Implantation. J Hepatol (2019) 70:50–7. 10.1016/j.jhep.2018.10.005
22.
De Carlis R Di Sandro S Lauterio A Botta F Ferla F Andorno E et al Liver Grafts from Donors after Circulatory Death on Regional Perfusion with Extended Warm Ischemia Compared with Donors after Brain Death. Liver Transpl (2018) 24:1523–35. 10.1002/lt.25312
23.
Dondossola D Ravaioli M Lonati C Maroni L Pini A Accardo C et al The Role of Ex Situ Hypothermic Oxygenated Machine Perfusion and Cold Preservation Time in Extended Criteria Donation after Circulatory Death and Donation after Brain Death. Liver Transpl (2021) 27:1130–43. 10.1002/lt.26067
24.
Ghinolfi D Dondossola D Rreka E Lonati C Pezzati D Cacciatoinsilla A et al Sequential Use of Normothermic Regional and Ex Situ Machine Perfusion in Donation after Circulatory Death Liver Transplant. Liver Transpl (2021) 27:385–402. 10.1002/lt.25899
25.
De Carlis R Schlegel A Frassoni S Olivieri T Ravaioli M Camagni S et al How to Preserve Liver Grafts from Circulatory Death with Long Warm Ischemia? A Retrospective Italian Cohort Study with Normothermic Regional Perfusion and Hypothermic Oxygenated Perfusion. Transplantation (2021) 105:2385–96. 10.1097/tp.0000000000003595
26.
Dutkowski P Oberkofler CE Slankamenac K Puhan MA Schadde E Müllhaupt B et al Are There Better Guidelines for Allocation in Liver Transplantation? Ann Surg (2011) 254:745–54. 10.1097/sla.0b013e3182365081
27.
Halldorson JB Bakthavatsalam R Fix O Reyes JD Perkins JD . D-MELD, a Simple Predictor of Post Liver Transplant Mortality for Optimization of Donor/Recipient Matching. Am J Transpl (2009) 9:318–26. 10.1111/j.1600-6143.2008.02491.x
28.
Zanierato M Dondossola D Palleschi A Zanella A . Donation after Circulatory Death: Possible Strategies for In-Situ Organ Preservation. Minerva Anestesiol (2020) 86:984–91. 10.23736/S0375-9393.20.14262-7
29.
De Carlis R Di Sandro S Lauterio A Ferla F Dell'Acqua A Zanierato M et al Successful Donation after Cardiac Death Liver Transplants with Prolonged Warm Ischemia Time Using Normothermic Regional Perfusion. Liver Transpl (2017) 23:166–73. 10.1002/lt.24666
30.
Aggarwal S Kang Y Freeman JA Fortunato FL Pinsky MR . Postreperfusion Syndrome: Cardiovascular Collapse Following Hepatic Reperfusion during Liver Transplantation. Transpl Proc (1987) 19:54–5.
31.
Hilmi I Horton CN Planinsic RM Sakai T Nicolau-Raducu R Damian D et al The Impact of Postreperfusion Syndrome on Short-Term Patient and Liver Allograft Outcome in Patients Undergoing Orthotopic Liver Transplantation. Liver Transpl (2008) 14:504–8. 10.1002/lt.21381
32.
Olthoff KM Kulik L Samstein B Kaminski M Abecassis M Emond J et al Validation of a Current Definition of Early Allograft Dysfunction in Liver Transplant Recipients and Analysis of Risk Factors. Liver Transpl (2010) 16:943–9. 10.1002/lt.22091
33.
Khwaja A . KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron (2012) 120:c179–c184. 10.1159/000339789
34.
Dindo D Demartines N Clavien P-A . Classification of Surgical Complications. Ann Surg (2004) 240:205–13. 10.1097/01.sla.0000133083.54934.ae
35.
Slankamenac K Graf R Barkun J Puhan MA Clavien P-A . The Comprehensive Complication Index. Ann Surg (2013) 258:1–7. 10.1097/sla.0b013e318296c732
36.
de Vries Y von Meijenfeldt FA Porte RJ . Post-transplant Cholangiopathy: Classification, Pathogenesis, and Preventive Strategies. Biochim Biophys Acta (Bba) - Mol Basis Dis (2018) 1864:1507–15. 10.1016/j.bbadis.2017.06.013
37.
Haque O Yuan Q Uygun K Markmann JF . Evolving Utilization of Donation after Circulatory Death Livers in Liver Transplantation: The Day of DCD Has Come. Clin Transpl (2021) 35:e14211. 10.1111/ctr.14211
38.
Research NBaT-SaC. Organ and Tissue Donation and Transplantation Activity Report 2020/21. NHS Blood and Transplant (2021). Available from: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/24053/activity-report-2020-2021.pdf (Accessed on January 10, 2022).
39.
Jochmans I Hessheimer AJ Neyrinck AP Paredes D Bellini MI Dark JH et al Consensus Statement on Normothermic Regional Perfusion in Donation after Circulatory Death: Report from the European Society for Organ Transplantation's Transplant Learning Journey. Transpl Int (2021) 34:2019–30. 10.1111/tri.13951
40.
De Beule J Vandendriessche K Pengel LHM Bellini MI Dark JH Hessheimer AJ et al A Systematic Review and Meta‐analyses of Regional Perfusion in Donation after Circulatory Death Solid Organ Transplantation. Transpl Int (2021) 34:2046–60. 10.1111/tri.14121
41.
Gregorini M Ticozzelli E Abelli M Grignano MA Pattonieri EF Giacomoni A et al Kidney Transplants from Donors on Extracorporeal Membrane Oxygenation Prior to Death Are Associated with Better Long-Term Renal Function Compared to Donors after Circulatory Death. Transpl Int (2021) 35:10179. 10.3389/ti.2021.10179
42.
Dutkowski P Polak WG Muiesan P Schlegel A Verhoeven CJ Scalera I et al First Comparison of Hypothermic Oxygenated PErfusion versus Static Cold Storage of Human Donation after Cardiac Death Liver Transplants. Ann Surg (2015) 262:764–71. 10.1097/sla.0000000000001473
43.
Dutkowski P Schlegel A de Oliveira M Müllhaupt B Neff F Clavien P-A . HOPE for Human Liver Grafts Obtained from Donors after Cardiac Death. J Hepatol (2014) 60:765–72. 10.1016/j.jhep.2013.11.023
44.
Op den Dries S Sutton ME Karimian N de Boer MT Wiersema-Buist J Gouw ASH et al Hypothermic Oxygenated Machine Perfusion Prevents Arteriolonecrosis of the Peribiliary Plexus in Pig Livers Donated after Circulatory Death. PLoS One (2014) 9:e88521. 10.1371/journal.pone.0088521
45.
Justo I Nutu A García-Conde M Marcacuzco A Manrique A Calvo J et al Incidence and Risk Factors of Primary Non-function after Liver Transplantation Using Grafts from Uncontrolled Donors after Circulatory Death. Clin Transpl (2021) 35:e14134. 10.1111/ctr.14134
46.
Valero R García-Valdecasas JC Tabet J Taurá P Rull Rn. Beltran J et al Hepatic Blood Flow and Oxygen Extraction Ratio during Normothermic Recirculation and Total Body Cooling as Viability Predictors in Non-heart-beating Donor Pigs1. Transplantation (1998) 66:170–6. 10.1097/00007890-199807270-00005
47.
Kalisvaart M Croome KP Hernandez-Alejandro R Pirenne J Cortés-Cerisuelo M Miñambres E et al Donor Warm Ischemia Time in DCD Liver Transplantation-Working Group Report from the ILTS DCD, Liver Preservation, and Machine Perfusion Consensus Conference. Transplantation (2021) 105:1156–64. 10.1097/tp.0000000000003819
48.
Bruggenwirth IMA van Leeuwen OB Porte RJ Martins PN . The Emerging Role of Viability Testing during Liver Machine Perfusion. Liver Transpl (2021) 2021. 10.1002/lt.26092
Summary
Keywords
donation after circulatory death, abdominal normothermic regional perfusion, hypothermic oxygenated machine perfusion, warm ischemia time, ischemic cholangiopathy, liver transplantation outcome
Citation
Patrono D, Zanierato M, Vergano M, Magaton C, Diale E, Rizza G, Catalano S, Mirabella S, Cocchis D, Potenza R, Livigni S, Balagna R and Romagnoli R (2022) Normothermic Regional Perfusion and Hypothermic Oxygenated Machine Perfusion for Livers Donated After Controlled Circulatory Death With Prolonged Warm Ischemia Time: A Matched Comparison With Livers From Brain-Dead Donors. Transpl Int 35:10390. doi: 10.3389/ti.2022.10390
Received
28 January 2022
Accepted
31 March 2022
Published
22 April 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Patrono, Zanierato, Vergano, Magaton, Diale, Rizza, Catalano, Mirabella, Cocchis, Potenza, Livigni, Balagna and Romagnoli.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renato Romagnoli, renato.romagnoli@unito.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.