Dear Editors,
Lung transplantation (LT) is accompanied by pro-inflammatory cytokine release, which correlates with the graft outcome (1–3). Extracorporeal cytokine adsorption therapy (ECAT) by Cytosorb® (CytoSorbents Corporation, Monmouth Junction, United States), a porous polymer beads adsorption cartridge, removes hydrophobic substances of molecular weight ≤60 kDa from the blood. ECAT is a promising therapy in hyperinflammatory situations (4–8), but has never been evaluated in LT. We evaluate for the first time ECAT on both circulating and membrane phagocyte-expressed inflammation biomarkers in the postoperative course of LT.
We conducted a prospective study at Bichat-Claude Bernard Hospital (Paris, France). Consecutive patients undergoing LT and admitted to the intensive care unit (ICU) postoperatively with extracorporeal membrane oxygenation (ECMO) were assessed. Cytosorb® cartridge was integrated into a bypass of the ECMO circuit at ICU admission. ECAT was performed during 24 h with the same cartridge. Blood samples were collected before cartridge placement (T0), after 24 h of ECAT (T1), and 24 h after cartridge removal (T2). We studied the evolution of membrane activation markers of neutrophils (CD66b and CD11b) and monocytes (CD14 and HLA-DR) by flow cytometry (Becton-Dickinson, FACS Lyric), the quantification of plasma levels of IL-6 and IL-8 by Luminex assay (Procartaplex®, Thermofisher) and L-lactate (Radiometer ABL90), and coagulation factors (factors II, V, VII, X, C protein, antithrombin III, and fibrinogen. Clinical data and outcomes are expressed in median (IQR). The study was approved by the French National Ethics Committee “Comité de Protection des Personnes Sud-Est II” (2017-A02625-48).
Six patients were transplanted for fibrosis (n = 4), chronic obstructive pulmonary disease (COPD) (n = 1) and silicosis (n = 1). At T2, neutrophil activation markers CD66b and CD11b expressions were significantly decreased as well as L-lactate levels (Figure 1). A downward trend was observed for monocyte activation markers (CD14 and HLA-DR), IL-6 and IL-8. No rebound effect was observed for any of these markers 24 h after cartridge removal. Coagulation markers were not altered. However, we observed one case of cartridge clotting after 12 h of treatment, without any consequences on the ECMO circuit. At T0, T1 and T2, norepinephrine doses were 0.75 (0.3–1.1), 0.25 (0.04–0.58) and 0.25 (0.03–1.15) μg/kg/min and PaO2/FiO2 ratio were 77 (74–118), 93 (88–107) and 79 (72–98) mmHg, respectively. Compared with a “control” cohort of 27 transplant patients over the same study period, the ICU length of stay and in hospital were longer for patients with ECAT, respectively of 64 (46–69) vs 41 (33–53) and 121 (82–146) vs 45 (38–63) days. However, at 1 year after LT, patients with ECAT were all alive, whereas the survival rate for patients in the “control” cohort without ECAT was 70.4%.
FIGURE 1
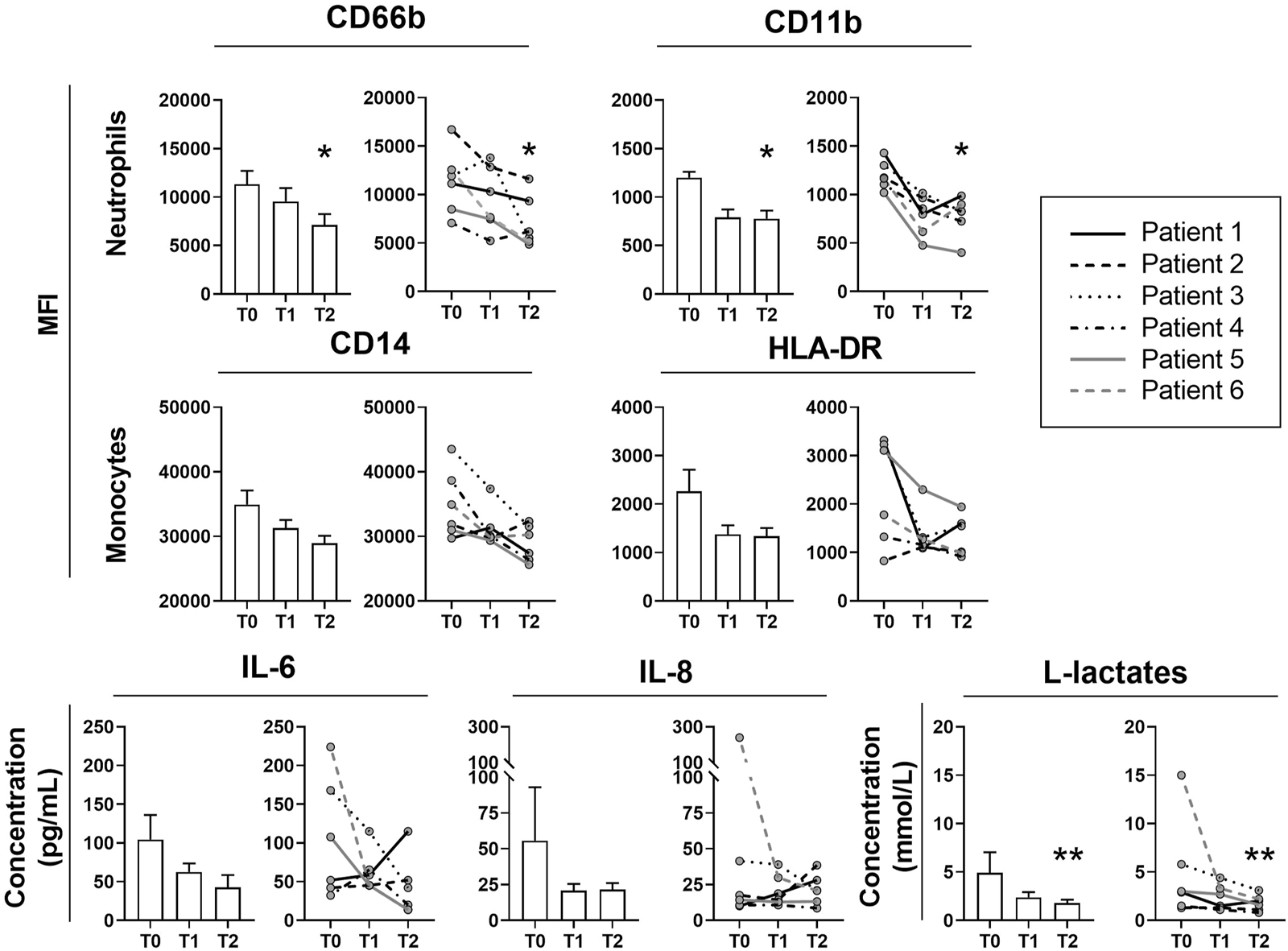
Evolution of neutrophil and monocyte membrane activation markers, IL-6, IL-8 and L-lactate during extracorporal cytokine adsorption therapy by Cytosorb®. Activation membrane markers of neutrophils (CD66b and CD11b) and monocytes (CD14 and HLA-DR) were assessed by flow cytometry before cartridge placement (T0), after 24 h of ECAT (T1), and 24 h after cartridge removal (T2) and are expressed as mean fluorescence intensity (MFI). IL-6 and IL-8 were quantified by Luminex assay and L-lactates were assessed with Radiometer ABL90 Flex and concentrations are expressed in pg/ml and mmol/L respectively. Data are expressed in histograms (left) as the mean ± SEM or as individual values (right). Friedmann’s test and Dunn’s post-hoc tests are represented. *p < 0.05 **p < 0.01, as compared to T0.
We present the first pilot study on the feasibility and efficacy of ECAT after LT. The decrease in neutrophil and monocyte activation markers has never been reported before and suggests a possible indirect immunomodulatory effect of ECAT on phagocyte activation. The decreased plasma IL-6 and IL-8 concentrations was not significant. However, the three patients with elevated IL-6 and/or IL-8 levels at T0 experienced a dramatic decrease at T1. Cytosorb® appears to be a safe and promising device to fight post-LT inflammation, and should be re-evaluated in a larger study.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was approved by the French National Ethics Committee “Comité de Protection des Personnes Sud-Est II” (2017-A02625-48). The patients/participants provided their written informed consent to participate in this study.
Author contributions
LC, AT-D, SC-M and PM designed the study. AT-D, ST, BL, JC, JM, HM, YC, PM included the patients and collected samples. PF performed the placement of the Cytosorb® cartridge on the ECMO. MP and DF performed in vitro analysis. MP and LC analysed the results. AT-D and MP wrote the manuscript draft. AT-D, MP, LC, SC-M edited the manuscript, all authors approved the final manuscript.
Funding
This work was funding by a grant of the Cytosorb® Company.
Acknowledgments
Service d’Anesthésie-Réanimation: Dan Longrois, Alexandre Mignon, Aurélie Snauwaert, Parvine Tashk, Maksud Assadi, Jules Stern, Sacha Rozencwajg, Adnan El Kalai, Aurélie Gouel, Fabien Lion, Laura Soldan, Adela Harpan, Marie-Pierre Dilly, Yassine Rkik, Atanas Sabahov, Claire Depont, Elie Kantor, Laetitia Desplanque, Nils Carrara, Sonia Yung, Morgan Roue, Alexandra Younes, Charles Moulin, Lea Copelovici, Iulia Balcan, Emmanuelle Busch. Service de Pneumologie B et Transplantation Pulmonaire: Gaëlle Weisenburger, Vincent Bunel, Cendrine Godet, Mathilde Salpin, Tiphaine Goletto, Chahine Medraoui, Domitille Mouren, Charlotte Thibaut de Menonville, Armelle Marceau, Gilles Jebrak, Lise Morer, Sabrina Trigueiros, Lucie Genet, Alice Savary, Zohra Brouk, Gwenn Frere, Agnès Abadie, Diego Ferreira, Sandrine Tissot. Service de Chirurgie Vasculaire, Thoracique et Transplantation Pulmonaire: Arnaud Roussel, Quentin Pellenc, Jean Senemaud, Iannis Ben Abdallah, Pierre Cerceau, Regis Renard.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
COPD, chronic obstructive pulmonary disease; ECAT, extracorporeal cytokine adsorption therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; LT, lung transplantation.
References
1.
Pham SM Yoshida Y Aeba R Hattler BG Iwaki Y Zeevi A et al Interleukin-6, a Marker of Preservation Injury in Clinical Lung Transplantation. J Heart Lung Transpl (1992) 11(6):1017–24.
2.
De Perrot M Sekine Y Fischer S Waddell TK McRae K Liu M et al Interleukin-8 Release during Early Reperfusion Predicts Graft Function in Human Lung Transplantation. Am J Respir Crit Care Med (2002) 165(2):211–5. 10.1164/ajrccm.165.2.2011151
3.
Mal H Dehoux M Sleiman C Boczkowski J Lesèche G Pariente R et al Early Release of Proinflammatory Cytokines after Lung Transplantation. Chest (1998) 113(3):645–51. 10.1378/chest.113.3.645
4.
Hawchar F Rao C Akil A Mehta Y Rugg C Scheier J et al The Potential Role of Extracorporeal Cytokine Removal in Hemodynamic Stabilization in Hyperinflammatory Shock. Biomedicines (2021) 9(7):768. 10.3390/biomedicines9070768
5.
Kogelmann K Jarczak D Scheller M Drüner M . Hemoadsorption by CytoSorb in Septic Patients: A Case Series. Crit Care (2017) 21(1):74. 10.1186/s13054-017-1662-9
6.
Kanjo A Molnar Z Zádori N Gede N Erőss B Szakó L et al Dosing of Extracorporeal Cytokine Removal in Septic Shock (DECRISS): Protocol of a Prospective, Randomised, Adaptive, Multicentre Clinical Trial. BMJ Open (2021) 11(8):e050464. 10.1136/bmjopen-2021-050464(
7.
Träger K Fritzler D Fischer G Schröder J Skrabal C Liebold A et al Treatment of Post-Cardiopulmonary Bypass SIRS by Hemoadsorption: A Case Series. Int J Artif Organs (2016) 39(3):141–6. 10.5301/ijao.5000492
8.
Tomescu DR Dima SO Ungureanu D Popescu M Tulbure D Popescu I . First Report of Cytokine Removal Using CytoSorb in Severe Noninfectious Inflammatory Syndrome after Liver Transplantation. Int J Artif Organs (2016) 39(3):136–40. 10.5301/ijao.5000489
Summary
Keywords
lung transplantation, hemoadsorption, cytokine, Cytosorb, inflammation
Citation
Peyneau M, de Chaisemartin L, Faille D, Messika J, Mal H, Castier Y, Mordant P, Carrasco JL, Tanaka S, Lortat Jacob B, Ferrari P, Arrault X, Ajzenberg N, Chollet-Martin S, Montravers P and Tran-Dinh A (2022) First Experience With Extracorporeal Cytokine Adsorption Therapy After Lung Transplantation. Transpl Int 35:10319. doi: 10.3389/ti.2022.10319
Received
21 December 2021
Accepted
14 February 2022
Published
21 March 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Peyneau, de Chaisemartin, Faille, Messika, Mal, Castier, Mordant, Carrasco, Tanaka, Lortat Jacob, Ferrari, Arrault, Ajzenberg, Chollet-Martin, Montravers and Tran-Dinh.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexy Tran-Dinh, alexy.trandinh@aphp.fr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.