Abstract
The predictive value of a subjective difficulty scale (DS) after surgical procedures is unknown. The objective of this study was to evaluate the prognostic value of a DS after liver transplantation (LT) and to identify predictors of difficulty. Surgeons prospectively evaluated the difficulty of 441 consecutive liver transplantations from donation after brain death at the end of the surgery by using a DS from 0 to 10 (“the easiest to the hardest you can imagine”). DS was associated with severe morbidity. The risk of graft loss at 1 year remained unchanged from 0 to 6 but increased beyond 6. Graft survival and patient survival of group with DS 7–10 was significantly impaired compared to groups with DS: 0–3 or DS: 4–6 but were significantly impaired for the group with DS: 7–10. Independent predictors of difficult LT (DS ≥ 7) were annular segment 1, transjugular intrahepatic portosystemic shunt, retransplantation beyond 30 days, portal vein thrombosis, and ascites. Of them, ascites was a borderline non-significant covariate (p = .04). Vascular complications occurred more often after difficult LT (20.5% vs. 5.9%), whereas there was no difference in the other types of complications. DS can be used to tailor monitoring and anticipate early complications. External validation is needed.
Introduction
The difficulty in achieving a surgical procedure dramatically varies from one patient to another, independently of its intrinsic complexity (1–5). Several difficulty scoring systems have been published in various surgical fields. These scores are usually built using surrogates of difficulty like blood loss or operation time (3, 6–8), or after selecting risk factors according to expert opinions (4, 5, 9).
This study focused on the technical difficulty of liver transplantation (LT) and proposed a different approach for assessing difficulty. Surgeons prospectively evaluated the difficulty by using a scale ranging from 0 to 10, according to their feeling at the end of the LT.
The prognostic value of such a subjective difficulty scale (DS) is unknown. Balance of Risk (BAR) and Survival Outcomes following Liver Transplantation (SOFT) scores are two validated tools that predict early survival after LT (10, 11). Both include donor and recipient pretransplant variables and cold ischemia time as the unique intraoperative parameter. We hypothesized that the performance of these scores could be improved by adding a subjective DS.
The objectives of this study were to test the impact of DS on outcomes and its added value with regard to validated prognostic models. Lastly, we aimed at identifying preoperative variables that predict difficult LT.
Patients and Methods
Study Population and Design
This study included all consecutive patients who underwent LT with a full liver graft from donation after brain death from January 2015 to March 2019 at the Paul Brousse Hospital, Villejuif, France. Every LT involved a fellow, defined here as a “junior” surgeon, and an attending defined as a “senior” surgeon. At the end of each LT, junior surgeons were in charge of entering intraoperative data into a dedicated online questionnaire, including a DS item. Junior surgeons were to give a number ranging from 0 to 10 (0 being the “easiest LT that you can imagine” and 10 being the “most difficult LT you can imagine”).
From October 2018 until the end of the study period, both senior and junior surgeons were asked to evaluate the DS, blinded for the evaluation of each other.
LTs without DS were not included. Donor variables were retrieved from the Cristal database of the Agence de la Biomédecine, the French national agency in charge of organ allocation. The design of this study was discussed and approved at our weekly institutional research meeting. This study was achieved in accordance with French legal requirements and the Declaration of Helsinki. Before surgery, patients provided their written consent according to which they permit that data obtained during standard health care can be used for scientific purposes.
CT Scan Review
Pretransplant CT scans were reviewed by YK, blinded for outcomes and DS value. The presence of the following items was assessed:
- annular segment I, defined as a complete inferior vena cava encirclement by hypertrophic caudal lobe.
- significant spontaneous portosystemic shunt (SPSS) ≥ 7 mm in diameter.
Technical Aspect of Liver Transplantation
Total hepatectomy was achieved with caval preservation and transient porto-caval anastomosis in most recipients. The caval anastomosis was done according to the three vein-piggy back technique (12). In the case of huge native liver, or annular segment 1, caval replacement was the preferred option. Portal inflow was obtained with a porto-portal termino-terminal anastomosis. PV thrombectomy was performed when necessary. In the case of a large spleno-renal shunt, left renal ligation or reno-portal anastomosis were decided according to the possibility of using the native portal vein (13). Extra-anatomical PV anastomosis was considered as the last option. For arterial reconstruction, hepatic artery with gastro-duodenal bifurcation was the option of choice.
Postoperative Management
Initial immunosuppression comprised a triple-drug regimen of tacrolimus, mycophenolate mofetil, and corticosteroid. Steroid boluses were used to treat moderate to severe acute rejection episodes after histological documentation. In selected cases, everolimus was introduced to enable early withdrawal of tacrolimus (14). An injected CT scan on day seven was performed routinely to detect vascular abnormalities (15). The post-transplant management and monitoring were done according to our local protocols regardless of the DS.
Statistical Analysis
All statistical analyses were performed using R version 3.5.1.
General overview
Our analysis followed 6 steps:
Step 1: We tested the relationship between DS and severe morbidity and 1-year patient survival.
Step 2: We evaluated the additional predictive value of DS by comparing the performance of BAR and SOFT scores before and after adding the DS.
Step 3: We compared survival according to three levels of difficulty: “easy” (0–3), “intermediate” (4–6), and “difficult” (7–10). Cutoff values to define these categories were arbitrarily chosen.
Step 4: We performed a univariate and multivariate analysis for predicting difficult transplantation.
Step 5: We compared the type of complications according to difficult transplantation.
Step 6: We tested the senior-junior agreement of DS during hepatectomy and implantation.
Methodology
In step 1, the relationship between DS and severe morbidity and 1-year patient survival was explored by using regression and Cox models, respectively.
DS was treated not as an ordinal variable but as a continuous variable for simplicity. Severe morbidity was defined by at least one grade IIIa event according to the Dindo-Clavien classification (16). Since several individuals have evaluated the DS, we sought for the possibility of subject-specific correlation. We tested whether the variable “individuals evaluating the DS” should be considered as a random or fixed variable (lremTest package) in the regression model. No significant random effect for this variable was detected, which led us to abandon mixed effect models. We left the variable “individuals assessing DS” in the logistic regression and Cox models as a covariate for more robustness (rms packages). Restricted cubic splines were used to relax from the linearity assumption (17). The assumption of proportionality of the Cox model was verified with Schoenfeld residuals.
In step 2, we evaluated the performance of the models without and with DS by using the Area Under Curve (AUC) and Akaike Information Criterion (AIC).
In step 3, graft survival was calculated from the date of LT. Data were censored at the time of last follow-up. The event of interest for graft survival was death or retransplantation, whereas death was the only event of interest used for patient survival calculation. Of note, for 1-year patient survival calculation, patients who died after 1 year from LT were censored. Survival curves were plotted according to Kaplan-Meier method. Survival probabilities were compared by using the log-rank test (ggplot2 packages).
In univariate analysis (step 4 and 5), continuous variables were expressed as median (range) and compared with the non-parametric Mann-Whitney test. Categorical variables were evaluated using chi-squared or Fisher exact tests, as appropriate. Variables associated with difficult transplant (p < .10) were entered into a multivariate regression model. The final choice of the model was guided according to the lowest AIC.
In step 6, we used the Lin concordance correlation coefficient (18) (DescTools package) to assess the agreement between junior and senior surgeons.
Results
Of the 631 LT performed during the study period, 525 LT met the inclusion criteria, i.e., a whole liver graft from donation after brain death. After excluding LT without available DS (n = 84, 16%), we obtained a study population of 441 LT, including a primary LT in 371 cases and retransplantation in the 70 remaining cases. During the study period, 404 patients underwent a single LT, 17 required two LTs, and one patient was transplanted three times, which represents a total of 422 patients.
For our study population, the 3-month and 1-year graft survival were 93% and 87%, respectively. One-year patient survival was 91%. Severe morbidity occurred in 166 (37.6%) LTs. A primary non-function was observed in 16 cases (3.6%).
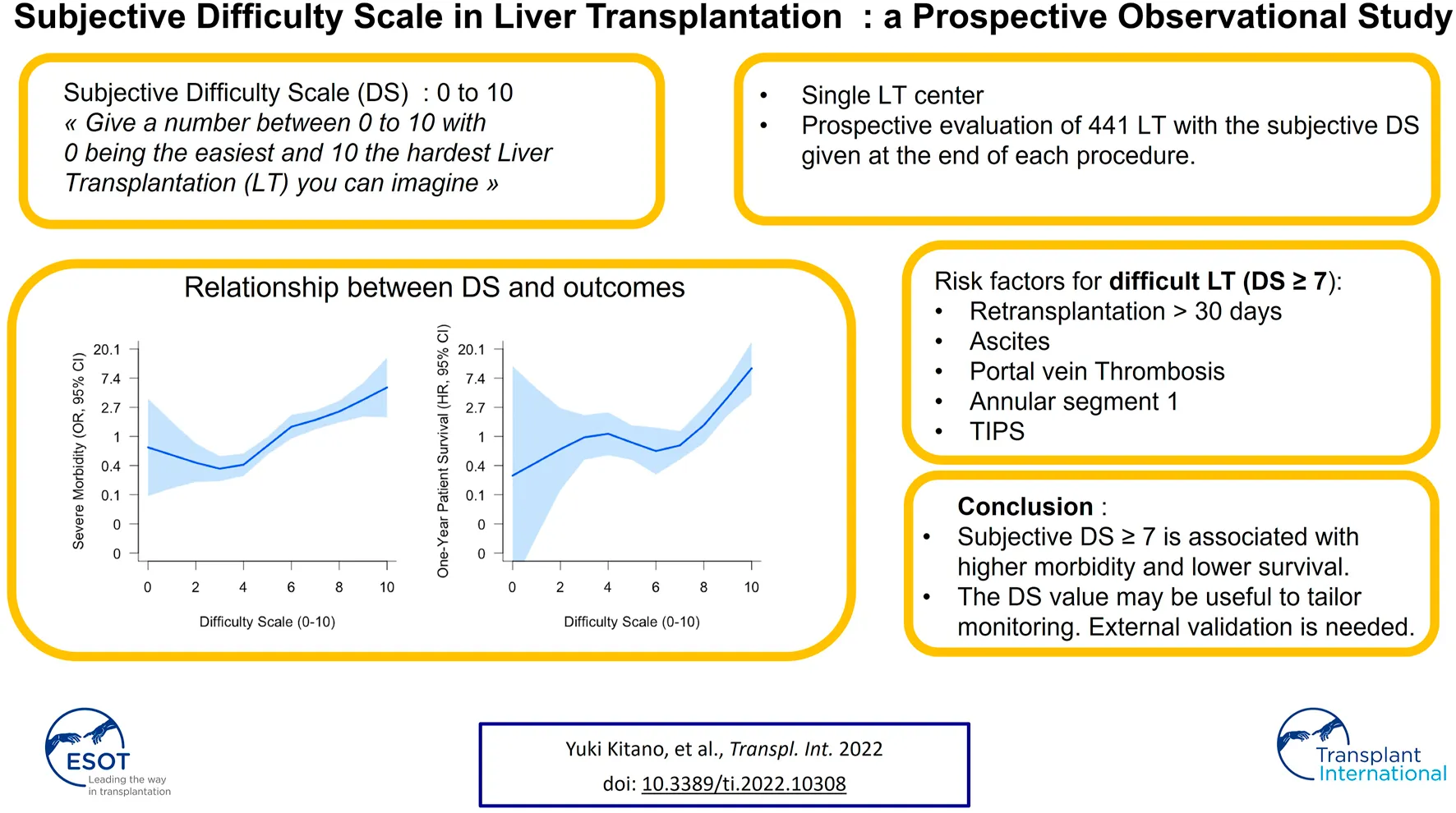
The DS was evaluated by twelve junior surgeons. The median value of DS was 6, ranging from 1 to 10. DS was comprised between 0–3, 4-6, and 7–10 in 66 (15%), 204 (46.3%), and 171 (38.8%) LTs, respectively. The distribution of DS values is shown in Figure 1.
FIGURE 1
Distribution of DS value across the study population.
Association Between Difficulty Scale Value and Severe Morbidity and One-Year Survival
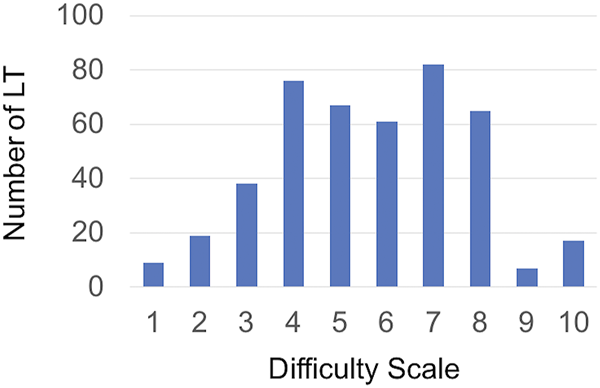
As shown in Figure 2, a continuous increase in the risk of severe morbidity as the DS increases was observed. In contrast, the hazard risk of death within the first year remained stable from 0 to 5 and started to increase from 6 to beyond.
FIGURE 2
Risk for severe morbidity (A) and 1-year patient survival (B) according to DS values. Shaded regions indicate 95% confidence bands. HR, Hazard Ratio; OR, Odds Ratio.
Additional Predictive Value of Difficulty Scale
The predictive value of BAR and SOFT models are given in Table 1. An increase of AUC and a decrease of AIC for all models were observed when adding the DS. The AUC of the models (with and without DS) were compared, and tests were significant for each model, indicating that DS improves the predictive value of each model for severe morbidity, 3-month graft survival, and 1-year graft survival.
TABLE 1
| Severe morbidity | |||||||
|---|---|---|---|---|---|---|---|
| Model | Variables | OR | 95% CI | p | AUC | AIC | p a |
| One-variable model | SOFT | 1.06 | 1.03–1.09 | <.001 | .63 | 545 | |
| Two-variable model | SOFT | 1.06 | 1.03–1.08 | <.001 | .721 | 510 | |
| DS | 1.40 | 1.26–1.57 | <.001 | <.001 | |||
| One-variable model | BAR | 1.08 | 1.04–1.12 | <.001 | .619 | 549 | |
| Two-variable model | BAR | 1.07 | 1.05–1.13 | <.001 | .727 | 510 | |
| DS | 1.48 | 1.30–1.64 | <.001 | ||||
| 3-months graft survival | |||||||
| Model | Variables | RR | 95% CI | p | AUC | AIC | p a |
| One-variable model | SOFT | 1.02 | 1.02–1.38 | .227 | .632 | 226 | |
| Two-variable model | SOFT + DS | 1.02 | 1.02–1.38 | .441 | .715 | 216 | <.001 |
| 1.38 | 1.14–1.70 | .001 | |||||
| One-variable model | BAR | 1.03 | .96–1.10 | .304 | .619 | 227 | |
| Two-variable model | BAR | 1.04 | .97–1.11 | .25 | .720 | 217 | <.001 |
| DS | 1.40 | 1.16–1.72 | <.001 | ||||
| One-year patient survival | |||||||
| Model | Variables | HR | 95% CI | p | AUC | AIC | p a |
| One-variable model | SOFT | 1.07 | 1.03–1.11 | <.001 | .664 | 407 | |
| Two-variable model | SOFT | 1.07 | 1.03–1.11 | .001 | .709 | 397 | <.001 |
| DS | 1.34 | 1.12–1.59 | .001 | ||||
| One-variable model | BAR | 1.08 | 1.2–1.14 | .007 | .626 | 412 | |
| Two-variable model | BAR | 1.08 | 1.2–1.14 | .008 | .701 | 399 | <.001 |
| DS | 1.39 | 1.17–1.66 | <.001 | ||||
Performance of SOFT and BAR models with and without DS for severe morbidity, 3-month graft survival, and 1-year patient survival.
Comparisons of AUC, with the roc. test function (pROC, package).
BAR; balance of risk; DS, difficulty scale; SOFT, survival outcomes after liver transplantation; OR, odds ratio.
Survival According to DS 0–3 vs. 4–6 vs. 7–10
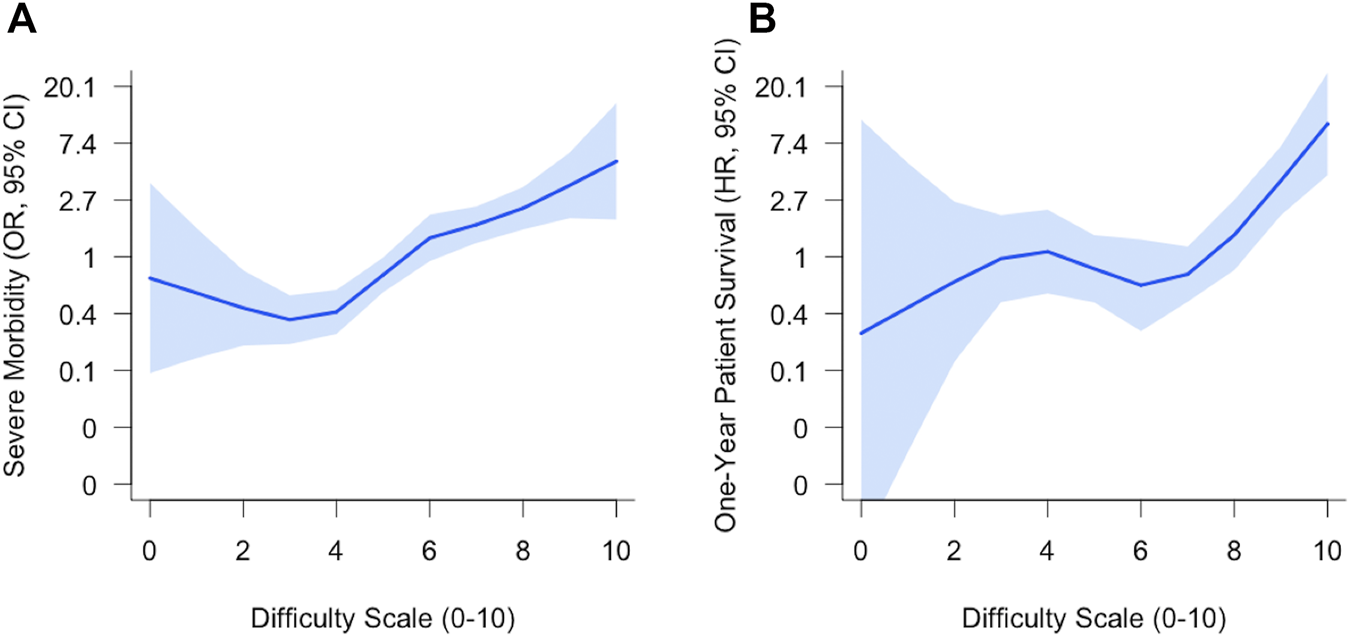
Graft survival and patient survival are reported in Figure 3. Graft survival of the group with DS ≥ 7 was significantly lower than graft survival with DS: 4-6 or DS: 0–3. Graft survival rates were 79% (95% CI: 73–85%), 91% (95% CI: 87%–95%), and 96% (95% CI: 93%–100%) at 1 year for the group DS: 7–10, DS: 4–6, and DS: 0–3, respectively. There was no difference between the two other groups DS 0–3 and DS: 4–6. Similar findings were observed for patient survival. One-year patient survival rates were 85% (95% CI: 82%–92%) in group DS 7–10 vs. 95% (95% CI: 92%–98%) and 97% (95% CI: 92%–100%) in the group with DS: 4–6 and DS: 0–3, respectively.
FIGURE 3
Kaplan-Meier curves for graft survival (A) and patient survival (B) according to DS 0–3 vs. 4–6 vs. 7–10.
Predictive Factors of Difficult LT (DS ≥ 7)
Univariate analysis is shown in Table 2. Transplant recipients with DS ≥ 7 had ascites, annular segment 1, PV thrombosis, or portal cavernoma more often. A previous transjugular intrahepatic portosystemic shunt (TIPS) was more present in this group. This group was also more likely to include ReLT > 30 days. The final multivariate model included five independent predictors of transplant with DS ≥ 7: previous TIPS (OR: 2.67 [1.06–7.11]), ascites (OR1.64 [1.07–2.51]), Portal Vein thrombosis (OR 2.17 [1.20–3.95]), annular segment 1 (OR 6.57 [2.71–18.48]), ReLT > 30 days (OR 4.11 [2.18–7.98] Table 3). Of note, ascites was a borderline non-significant variable in this multivariable model.
TABLE 2
| Variables | DS < 7 | DS ≥ 7 | p | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| N = 270 (range or %) | N = 171 (range or %) | OR | 95% CI | p | ||
| Recipient | ||||||
| Male Sex | 184 (68.1) | 128 (74.9) | .161 | |||
| Age, years | 55.0 (15.0–71.0) | 53.0 (12.0–71.0) | .300 | |||
| BMI, kg/m2 | 25.2 (15.4–45.7) | 25.1 (11.4–46.1) | .741 | |||
| MELD score at transplant | 19.0 (6.0–40.0) | 19.0 (6.0–40.0) | .516 | |||
| ICU at the time of transplant | 53 (19.6) | 29 (17.0) | .564 | |||
| Pretransplant dialysis | 12 (4.44) | 10 (5.85) | .663 | |||
| ReLT beyond 30 days | 17 (6.30) | 32 (18.8) | <.001 | 4.11 | 2.18–7.99 | <.001 |
| TIPS in place | 8 (2.96) | 16 (9.41) | .007 | 2.68 | 1.06–7.12 | .02 |
| Combined Kidney transplant | 16 (5.93) | 12 (7.02) | .797 | |||
| Explant weight, g | 1,295 (400–6,290) | 1,315 (435–3,665) | .532 | |||
| Pretransplant TACE | 53 (19.6) | 31 (18.2) | .812 | |||
| Night time (10 pm–6 am) | 43 (15.9) | 28 (16.4) | >.99 | |||
| Donor | ||||||
| Male sex | 142 (52.6) | 100 (58.5) | .266 | |||
| Age, years | 60.0 (6.00–91.0) | 57.0 (14.0–93.0) | .318 | |||
| BMI, kg/m2 | 24.7 (13.8–51.3) | 24.2 (14.6–41.0) | .595 | |||
| Weight of the graft, g | 1,332 (700–2,425) | 1,400 (685–2,795) | .168 | |||
| GW/recipient BW ratio | 1.8 (.7–4.3) | 1.8 (.8–5.9) | .601 | |||
| Explant weight/recipient BW ratio | 1.7 (.7–10.5) | 1.7 (.6–6.9) | .965 | |||
| Pretransplant CT scan | ||||||
| Ascitesa | 103 (39.0) | 95 (56.2) | .001 | 1.64 | 1.07–2.51 | .04 |
| Annular segment 1 | 6 (2.27) | 25 (14.9) | <.001 | 6.58 | 2.71–18.49 | <.001 |
| Annular segment 1 and Piggy Back caval anastomosis | 3 (1.1) | 17 (10.1) | <.001 | |||
| Portosystemic shunt | 120 (45.5) | 116 (69) | <.001 | |||
| Portal vein thrombosis | 25 (9.5) | 38 (22.6) | <.001 | 2.17 | 1.20–3.95 | .01 |
| PVT Yerdel 1–2b | 25 (9.5) | 30 (17.5) | <.001 | |||
| PVT Yerdel 3 | 0 (0) | 8 (4.8) | ||||
| Scoring systems | ||||||
| BAR | 8 (1–22) | 8 (1–22) | .571 | |||
| D-MELD | 1,050 (162–5,312) | 1,064 (153–3,400) | .387 | |||
| SOFT | 9 (3–36) | 12 (0–45) | .004 | |||
| ET-DRI | 1.47 (.95–2.86) | 1.44 (.97–2.71) | .938 | |||
Risk factors for difficult LT (DS ≥ 7): Univariable and multivariable logistic regression analysis.
BAR; balance of risk; BMI, body mass index; BW, body weight; D-MELD, Donor age X MELD, score; ET-DRI, European Transplant—Donor Risk Index; GW, graft weight; ICU, intensve care unit; MELD, Model for end-stage liver Disease; PVT, portal vein thrombosis; RBC, red blood cell; SOFT, survival outcomes following liver transplantation; TACE, transarterial chemoembolization; TIPS, transjugular intrahepatic portosystemic shunt.
Ascites was defined regardless of its volume, according to intraoperative finding at laparotomy.
Yerdel classification (30).
() indicates range for continuous variables and % for categorical variables.
TABLE 3
| Observed probability of DS ≥ 7 | |
|---|---|
| No. Factor | No. DS ≥ 7/overall number |
| 0 | 46/177 (26%) |
| 1 | 59/169 (35%) |
| 2 | 45/65 (69%) |
| 3+ | 18/21 (86%) |
Oberved probabilities for difficult LT (DS ≥ 7) according to the number of risk factors (Annular segment 1, ReLT after 30 days, Ascites, Portal vein thrombosis, TIPS).
Observed proportions of difficult transplant according to the number of factors are given in Table 3. It ranges from 26% to 86% in transplant without risk factors and at least three risk factors.
Complications Associated With Difficult Liver Transplantation
The type of surgical complications, according to LT difficulty DS < 7 vs. DS ≥ 7, is shown in Table 4. A higher proportion of vascular complications was observed after difficult LT (20.5% vs. 5.9%; p < .001). In contrast, there was no difference in the other types of surgical complications between the two groups. However, the proportion of renal failure tends to be higher in the difficult LT group (borderlin significance).
TABLE 4
| Type of complications | DS < 7 | DS ≥ 7 | p |
|---|---|---|---|
| N = 270 | N = 171 | ||
| Early allograft dysfunctiona | 57 (21.1%) | 49 (28.7%) | .091 |
| Vascular complicationsb | 16 (5.9%) | 35 (20.5%) | <.001 |
| Biliary complicationsc | 9 (3.3%) | 5 (2.9%) | >.99 |
| Hemorrhaged | 31 (11.5%) | 24 (14.0%) | .520 |
| Infection | 71 (26.3%) | 56 (32.7%) | .177 |
| Renal failuree | 18 (6.7%) | 21 (12.3%) | .064 |
Complications according to DS.
According to Olthoff et al.
Thrombosis or stenosis of the hepatic artery, the portal vein or caval anastomosis diagnosed on imaging regardless of the management.
Stenosis or biliary fistula.
Hemorrhage requiring laparotomy or hematoma on imaging requiring transfusion.
Stage III acute kidney injury (KDIGO Classification).
Agreement Between Junior and Senior Surgeons
The DS values given by the junior and senior are given in Figure 4. Diameters of points vary according to the number of evaluations. Points distributed on the diagonal line corresponds to perfect agreement. Points above the diagonal lines indicate that LT was considered more difficult by the senior surgeon, whereas points below refer to harder transplant from the junior point of view. Overall, the agreement was satisfactory. The concordance coefficient correlations (95% CI) were .65 (.51–.76) and .78 (.69–.86) for hepatectomy and implantation, respectively.
FIGURE 4
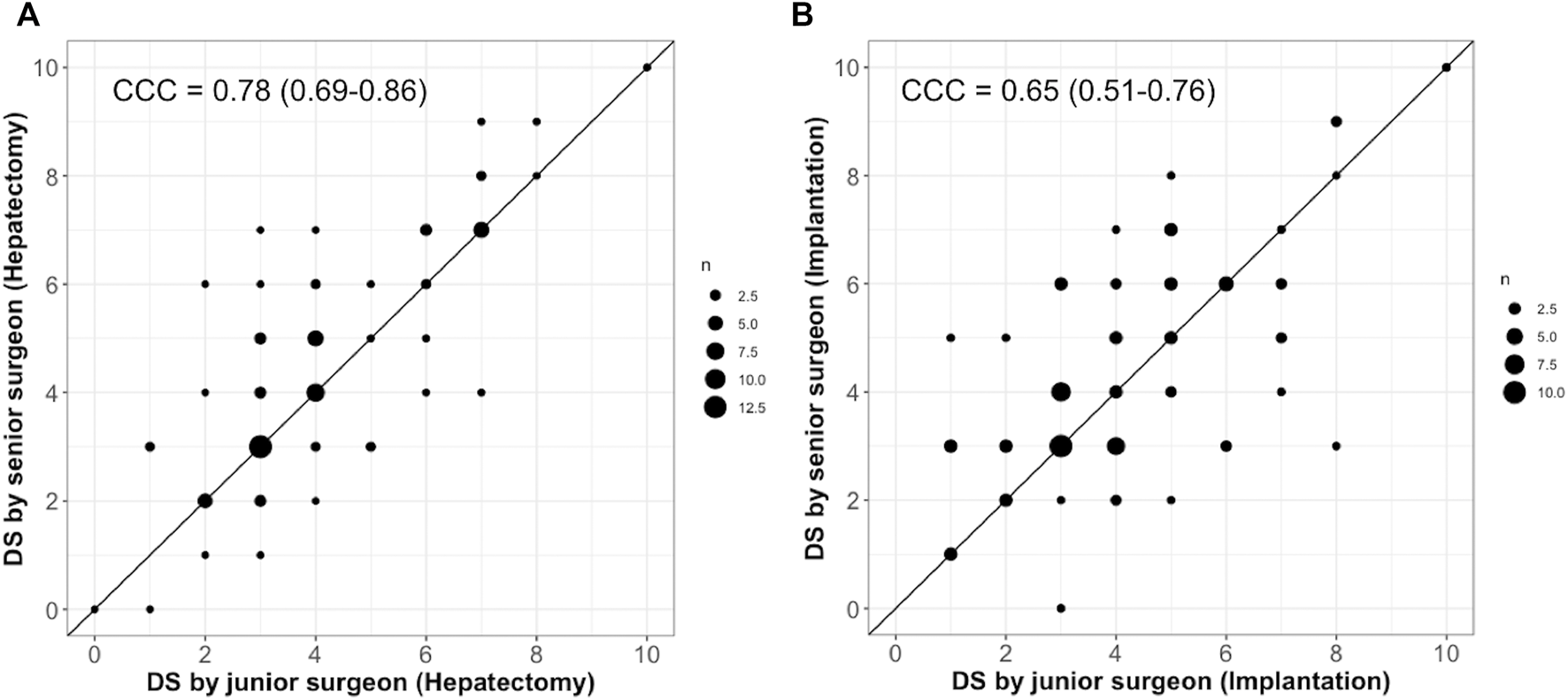
Agreement of DS between junior and senior surgeons for hepatectomy (A) and implantation (B).
Discussion
The technical difficulty is inherently subjective. In previous studies, the technical difficulty in surgery was assessed by using various surrogates. The originality of our study was to prospectively evaluate the difficulty according to the surgeon’s subjective feeling at the end of the transplantation.
We observed that DS correlates with morbidity and even 1-year survival. The importance of intraoperative factors to improve the predictive ability of pretransplant models has been recognized (19, 20). Adding the sole DS was sufficient to improve two validated pretransplant models, namely the BAR and SOFT scores, which means that DS should be not be used in lieu of these models but in conjunction.
As expected, the DS was associated with some objective variables like duration of surgery or transfusion volume, already known to impact outcomes (21, 22). The main strength of the DS is to reflect some subjective predictors of outcomes such as the surgical field exposure, the quality of tissues, and the easiness to achieve vascular or biliary anastomosis, which cannot be captured by usual metric tools. The DS can be seen as a summary of the numerous factors of difficulty, all contributing directly or indirectly to outcomes. This latter point may explain the predictive value of the DS.
The risk of death within the first year started to sharply increase beyond 6, suggesting that this cutoff value of seven carries a relevant clinical meaning. Five independent factors of “difficult” transplant were identified. Of them, late retransplantation is not a surprising finding. Adhesions, sometimes filled by portal hypertension, and modified anatomical landmark makes ReLT more challenging than primary transplantation (23, 24). A complete encirclement of the retrohepatic inferior vena cava is known to increase the difficulty and the risk of total hepatectomy with caval preservation (25). Preexisting TIPS is also associated with an increased risk of bleeding during total hepatectomy, especially in cases of misplacement (26). PV thrombosis may compromise the portal inflow, essential for graft function recovery. In most cases, eversion thrombectomy is sufficient to restore a sufficient portal flow. In the presence of a complete thrombosis of the PV and superior mesenteric vein, other more technically demanding strategies are needed to obtain adequate portal perfusion. The impossibility of restoring sufficient portal flow may force to consider technically demanding strategies, which consist of anastomosing the graft PV to the recipient superior mesenteric vein, gastric, choledochal varices, or left renal vein (27, 28).
Identifying “difficult” transplants with pretransplant variables yields some logistics advantages. Recipient laparotomy should begin as early as possible to limit cold ischemia time. DS highlights some technical difficulties such as annular segment 1 or portal vein thrombosis and may serve to better define the surgical strategy before LT. Complex transplantation may also require a team of two experienced surgeons. It may also guide the graft choice and avoid the combination of a marginal graft and complex transplantation associated with poor results (29).
The DS may also be of interest in the early post-transplant period. Some patients after “technically easy” LT are likely good candidates for enhanced recovery protocol, whereas recipients with high DS may potentially benefit from tailored monitoring, including daily Doppler and systematic CT scan. However, the possibility to tailor monitoring according to DS remains a hypothesis, and a more refined difficulty scale (evaluating each step, for example) might be a more efficient approach to anticipate outcomes.
The DS proposed here is prone to biases. An important variation in the evaluation according to experience, surgical skills, and timing of surgery could be expected. A surgeon’s “feeling at the end of LT” can be affected by innumerable variables, including the type of procedure, time of day, surgeon or assistant exhaustion or mood, issues with anesthesia, instruments, staff personnel, and many other factors, some even unrelated to surgical or medical aspects. As a result, the same case, potentially with the same outcome, could be subjectively evaluated by the surgeon differently in contrasting circumstances. In addition, the agreement across centers may not be warranted, depending on recruitment, number of cases, and type of disease treated. We also observed acceptable agreement between the senior and junior surgeon evaluations, suggesting that DS keeps a reasonable degree of reproducibility, despite its subjectivity. Discordant values in the DS were mainly observed in the intermediate range of difficulty, whereas “difficult” and “easy” were less subject to disagreement. The present study carries some limitations, in addition to its monocentric nature. The DS has not been evaluated in 16% of LT. We decided not to use multiple imputations because DS is the primary variable of interest. The comparisons of the study population with the group of LTs without DS showed significant differences for junior surgeons but neither for recipient characteristics nor intraoperative data.
The DS did not evaluate specifically for total hepatectomy and graft implantation in the whole cohort. A pretransplant DS would also have been helpful to test predictive variables and study the discrepancy between pre- and post-transplant DS. Validation of the DS prognostic value and the risk factors for complex transplant on an independent cohort is necessary to test the reproducibility and the relevancy of the DS in routine.
In conclusion, end-transplant DS predicts morbidity and 1-year survival after liver transplantation. Its value may be helpful to adapt monitoring and facilitate the early diagnosis of complications.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, under reasonable request.
Ethics statement
The design of this study was discussed and approved at our weekly institutional research meeting. This study was achieved in accordance with French legal requirements and the Declaration of Helsinki. Before surgery, patients have given a written consent according to which they permit that data obtained during standard health care can be used for scientific purposes.
Author contributions
YK and M-AA designed the study. YK, DP, EF-S, and M-AA performed the research. YK and M-AA wrote the manuscript. DC, DA, EV, RA, AC, HB, and NG contributed important reagents.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BAR, balance-of-risk; DS, difficulty scale; ET-DRI, Eurotransplant Donor Risk Index; ICU, intensive care unit; LT, liver transplantation; MELD, Model for End-stage Liver Disease; PV, Portal vein; ReLT, Retransplantation; RBC, red blood cells; TACE, transarterial chemoembolization; TIPS, transjugular intrahepatic portosystemic shunt; SOFT, survival outcomes following liver transplantation.
References
1.
Law KE Lowndes BR Kelley SR Blocker RC Larson DW Hallbeck MS et al Surgeon Workload in Colorectal Surgery: Perceived Drivers of Procedural Difficulty. J Surg Res (2020) 245:57–63. 10.1016/j.jss.2019.06.084
2.
Schwartz FR Shaw BI Lerebours R Vernuccio F Rigiroli F Gonzalez F et al Correlation of Preoperative Imaging Characteristics with Donor Outcomes and Operative Difficulty in Laparoscopic Donor Nephrectomy. Am J Transpl (2020) 20:752–60. 10.1111/ajt.15608
3.
Kawaguchi Y Fuks D Kokudo N Gayet B . Difficulty of Laparoscopic Liver Resection. Ann Surg (2018) 267:13–7. 10.1097/sla.0000000000002176
4.
Atallah MM Demian AD Shorrab AA . Development of a Difficulty Score for Spinal Anaesthesia. Br J Anaesth (2004) 92:354–60. 10.1093/bja/aeh073
5.
D’Orazi V Sacconi A Trombetta S Karpathiotakis M Pichelli D Di Lorenzo E et al May Predictors of Difficulty in Thyroid Surgery Increase the Incidence of Complications? Prospective Study with the Proposal of a Preoperative Score. BMC Surg (2019) 18. 10.1186/s12893-018-0447-7
6.
Krizzuk D Yellinek S Parlade A Liang H Dasilva G Wexner SD . A Simple Difficulty Scoring System for Laparoscopic Total Mesorectal Excision. Tech Coloproctol (2020) 24:1137–43. 10.1007/s10151-020-02285-8
7.
Halls MC Berardi G Cipriani F Barkhatov L Lainas P Harris S et al Development and Validation of a Difficulty Score to Predict Intraoperative Complications during Laparoscopic Liver Resection. Br J Surg (2018) 105:1182–91. 10.1002/bjs.10821
8.
Chen W Li Q Fan Y Li D Jiang L Qiu P et al Factors Predicting Difficulty of Laparoscopic Low Anterior Resection for Rectal Cancer with Total Mesorectal Excision and Double Stapling Technique. PLOS ONE (2016) 11:e0151773. 10.1371/journal.pone.0151773
9.
Ban D Tanabe M Ito H Otsuka Y Nitta H Abe Y et al A Novel Difficulty Scoring System for Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci (2014) 21:745–53. 10.1002/jhbp.166
10.
Dutkowski P Oberkofler CE Slankamenac K Puhan MA Schadde E Müllhaupt B et al Are There Better Guidelines for Allocation in Liver Transplantation. Ann Surg (2011) 254:745–54. 10.1097/sla.0b013e3182365081
11.
Rana A Hardy MA Halazun KJ Woodland DC Ratner LE Samstein B et al Survival Outcomes Following Liver Transplantation (SOFT) Score: A Novel Method to Predict Patient Survival Following Liver Transplantation. Am J Transplant (2008) 8:2537–46. 10.1111/j.1600-6143.2008.02400.x
12.
Tayar C Kluger MD Laurent A Cherqui D . Optimizing Outflow in Piggyback Liver Transplantation without Caval Occlusion: The Three-Vein Technique. Liver Transpl (2011) 17:88–92. 10.1002/lt.22201
13.
Golse N Bucur PO Faitot F Bekheit M Pittau G Ciacio O et al Spontaneous Splenorenal Shunt in Liver Transplantation. Transplantation (2015) 99:2576–85. 10.1097/tp.0000000000000766
14.
Saliba F Duvoux C Gugenheim J Kamar N Dharancy S Salamé E et al Efficacy and Safety of Everolimus and Mycophenolic Acid with Early Tacrolimus Withdrawal after Liver Transplantation: A Multicenter Randomized Trial. Am J Transpl (2017) 17:1843–52. 10.1111/ajt.14212
15.
Memeo R Ciacio O Pittau G Cherqui D Castaing D Adam R et al Systematic Computer Tomographic Scans 7 Days after Liver Transplantation Surgery Can Lower Rates of Repeat-Transplantation Due to Arterial Complications. Transplant Proc (2014) 46:3536–42. 10.1016/j.transproceed.2014.04.017
16.
Clavien PA Barkun J de Oliveira ML Vauthey JN Dindo D Schulick RD et al The Clavien-Dindo Classification of Surgical Complications. Ann Surg (2009) 250:187–96. 10.1097/sla.0b013e3181b13ca2
17.
Gauthier J Wu QV Gooley TA . Cubic Splines to Model Relationships between Continuous Variables and Outcomes: a Guide for Clinicians. Bone Marrow Transpl (2020) 55:675–80. 10.1038/s41409-019-0679-x
18.
Lin LI-K . A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics (1989) 45:255–68. 10.2307/2532051
19.
Han S Kwon JH Jung SH Seo JY Jo YJ Jang JS et al Perioperative Fresh Red Blood Cell Transfusion May Negatively Affect Recipient Survival after Liver Transplantation. Ann Surg (2018) 267:346–51. 10.1097/sla.0000000000002062
20.
Brüggenwirth IMA Dolgin NH Porte RJ Bozorgzadeh A Martins PNA . Donor Diabetes and Prolonged Cold Ischemia Time Synergistically Increase the Risk of Graft Failure after Liver Transplantation. Transplant Direct (2017) 3:e173. 10.1097/txd.0000000000000692
21.
Benson AB Burton JR Austin GL Biggins SW Zimmerman MA Kam I et al Differential Effects of Plasma and Red Blood Cell Transfusions on Acute Lung Injury and Infection Risk Following Liver Transplantation. Liver Transpl (2011) 17:149–58. 10.1002/lt.22212
22.
Rana A Petrowsky H Hong JC Agopian VG Kaldas FM Farmer D et al Blood Transfusion Requirement during Liver Transplantation Is an Important Risk Factor for Mortality. J Am Coll Surgeons (2013) 216:902–7. 10.1016/j.jamcollsurg.2012.12.047
23.
Maggi U Andorno E Rossi G De Carlis L Cillo U Bresadola F et al Liver Retransplantation in Adults: The Largest Multicenter Italian Study. PLoS ONE (2012) 7:e46643. 10.1371/journal.pone.0046643
24.
Yoo PS Umman V Rodriguez-Davalos MI Emre SH . Retransplantation of the Liver: Review of Current Literature for Decision Making and Technical Considerations. Transplant Proc (2013) 45:854–9. 10.1016/j.transproceed.2013.02.063
25.
Panaro F Boisset G Chanques G Guiu B Herrero A Bouyabrine H et al Vena Cava Encirclement Predicts Difficult Native Hepatectomy. Liver Transpl (2016) 22:906–13. 10.1002/lt.24478
26.
Addeo P Schaaf C Faitot F Terrone A Julliard O Besch C et al Misplacement of Transjugular Intrahepatic Portosystemic Shunts: A Surgical challenge for Liver Transplantation. Surgery (2021) 169:447–54. 10.1016/j.surg.2020.07.028
27.
Bhangui P Lim C Levesque E Salloum C Lahat E Feray C et al Novel Classification of Non-malignant portal Vein Thrombosis: A Guide to Surgical Decision-Making during Liver Transplantation. J Hepatol (2019) 71:1038–50. 10.1016/j.jhep.2019.08.012
28.
D'Amico G Hassan A Diago Uso T Hashmimoto K Aucejo FN Fujiki M et al Renoportal Anastomosis in Liver Transplantation and its Impact on Patient Outcomes: a Systematic Literature Review. Transpl Int (2019) 32:117–27. 10.1111/tri.13368
29.
Kalisvaart M Perera MTPR . Using Marginal Grafts for Liver Transplantation: The Balance of Risk. J Invest Surg (2020) 33:565–7. 10.1080/08941939.2018.1542048
30.
Yerdel MA Gunson B Mirza D Karayal??in K Olliff S Buckels J et al Portal Vein Thrombosis in Adults Undergoing Liver Transplantation. Transplantation (2000) 69:1873–81. 10.1097/00007890-200005150-00023
Summary
Keywords
liver transplantation, difficulty, subjective difficulty, technical difficulty, retransplantation
Citation
Kitano Y, Pietrasz D, Fernandez-Sevilla E, Golse N, Vibert E, Sa Cunha A, Azoulay D, Cherqui D, Baba H, Adam R and Allard M-A (2022) Subjective Difficulty Scale in Liver Transplantation: A Prospective Observational Study. Transpl Int 35:10308. doi: 10.3389/ti.2022.10308
Received
14 December 2021
Accepted
18 January 2022
Published
21 March 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Kitano, Pietrasz, Fernandez-Sevilla, Golse, Vibert, Sa Cunha, Azoulay, Cherqui, Baba, Adam and Allard.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc-Antoine Allard, marcantoine.allard@aphp.fr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.