Abstract
Purpose: Our previous study has demonstrated that tetracycline exerts excellent bactericidal activity against Staphylococcus aureus strains isolated from patients with atopic dermatitis (AD) while simultaneously inhibiting the development of T helper (Th) type 2 (Th2) cells. The present study was designed to evaluate the effectiveness of dual therapy with betamethasone and tetracycline for AD.
Methods: Betametasone (0.1%) and tetracycline (3%) were topically administered to NC/Nga mice with AD-like skin lesions. Skin severity scores, histological changes to the lesioned skin, and serum IgE levels were assessed as indicators of therapeutic effectiveness.
Results: Topical treatment with both drugs reduced the skin severity score more significantly than was the case with betamethasone alone or tetracycline alone. This was associated with a reduction in the degree of epidermal thickening, the density of cellular infiltration into the dermis, the mast cell count in the dermis and the serum IgE concentration. Furthermore, the degree of Th1/Th2 cell development in auricular lymph nodes and the S. aureus count on the lesioned skin were synergistically suppressed by simultaneous application of both drugs.
Conclusion: The present results show that simultaneous topical application of betamethasone and tetracycline synergistically ameliorates AD-like skin lesions in NC/Nga mice. This suggests that dual therapy with betamethasone and tetracycline for AD lesions colonized by S. aureus might be one of the best options for inhibiting the development of both Th1 and Th2 cells and acting on superficially located S. aureus.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease with immunopathologic features that vary depending on the duration of the skin lesions (1). Epidemiological studies have shown that AD significantly influences on the quality of life of patients, and its worldwide prevalence is estimated to be about 10%–30% in children and about 2%–10% in adults (2, 3). AD develops early in childhood, and its onset has been linked to multiple genetic abnormalities, a decrease of skin barrier function, immune response abnormality and various environmental factors (4). If the barrier function of the skin is defective, the immune response to the normal skin microbiome is disturbed, and this plays a major role in the pathogenesis of AD (5). The majority of AD patients show superficial skin colonization by the Gram-positive bacterium Staphylococcus aureus and increased expression of T helper (Th) type 2 (Th2) cytokines such as interleukin (IL)-4, IL-5 and IL-13 in their peripheral blood mononuclear cells or skin lesions (6, 7). S. aureus can be isolated from 96% to 100% of AD skin lesions, whereas only 0–10% of healthy individuals show skin colonization by this organism (8, 9). Furthermore, the frequency of S. aureus colonization in the lesional skin of AD patients is markedly higher than that in non-lesional skin, and the S. aureus bacterial cell count is also significantly higher in lesional skin than in non-lesional skin (9). We have previously demonstrated that chronic skin colonization with S. aureus may augment the development of Th2 cells in AD patients (10), and that S. aureus cell wall components induce an increase in the numbers of eosinophils, mast cells and Th2 cells, which are closely associated with exacerbation of skin inflammation in the lesional skin (11, 12, 13, 14). Therefore, any antibiotic effective for AD patients should ideally benefit not only those with impetiginized AD but also those without clinical signs of superinfection. In our previous study, we found that tetracycline exerted excellent bactericidal activity against S. aureus strains isolated from AD skin lesions and simultaneously suppressed Th2 cell development mediated by Langerhans cells (LCs), with effects equivalent to those of betamethasone (15, 16). Therefore, in the present study using a model of AD in NC/Nga mice, we evaluated the effect of dual therapy with betamethasone and tetracycline on AD lesions.
Methods
Mice
The mice used in this study were 6-week-old female specific pathogen-free NC/Nga mice obtained from Japan SLC (Hamamatsu, Japan). They were housed in plastic cages with sterilized paper bedding in a clean, air-conditioned room at 24°C and allowed free access to a standard laboratory diet and water. All procedures performed on the mice were in accordance with the guidelines of the Animal Care and Use Committee of Meiji Pharmaceutical University and approved by the committee.
Reagents
A mite antigen, Dermatophagoides farinae extract (Biostir AD), was purchased from Biostir Inc. (Osaka, Japan). Betamethasone and tetracycline were obtained from Tokyo Chemical Industry (Tokyo, Japan). White petrolatum including 5% (w/w) liquid paraffin was employed as the vehicle and used to prepare 0.1% (w/w) betamethasone ointment and 3% (w/w) tetracycline ointment.
Induction of AD-like skin lesions
The dorsal hair of NC/Nga mice was shaved with a hair clipper and finally removed with a depilatory cream. Skin barrier disruption was then achieved by topical application of 150 μL of 4% sodium dodecyl sulfate to the dorsal skin (120 μL/8 cm2 skin) and the bilateral auricle skin (15 μL/1 cm2 skin). After 3 h, 100 mg Biostir AD was applied topically to the dorsal skin (80 mg/8 cm2 skin) and the bilateral auricle skin (10 mg/1 cm2 skin). These procedures were repeated every 3 or 4 days. The design of the experimental schedule is shown in Figure 1.
FIGURE 1
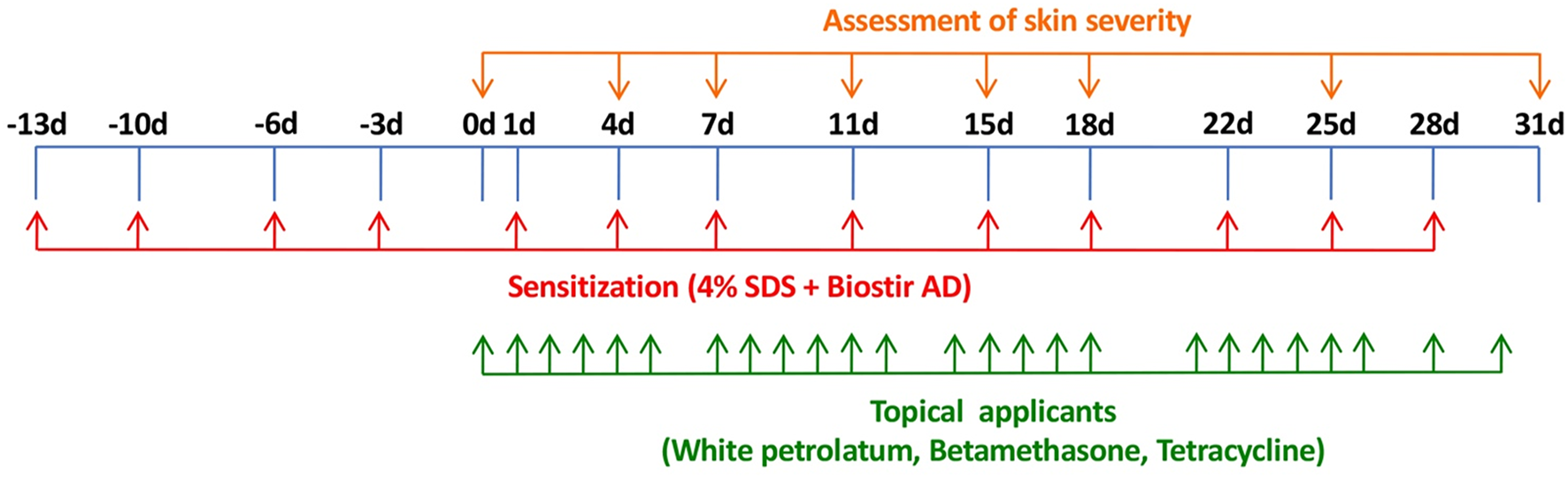
Experimental schedule for induction of AD-like dermatitis in NC/Nga mice and treatment with betamethasone and tetracycline ointment.
Measurement of skin severity score
Dermatitis severity was assessed macroscopically using the scoring system described previously (17). Briefly, one skin lesion (1 cm2) on each ear and one skin lesion (8 cm2) on the back were scored on the basis of the following criteria. The dermatitis score (minimum 0; maximum 30 [=3 regions × 2 points × 5 symptoms]) was calculated as the sum of the individual scores for the three regions, and graded as 0 (no symptoms), 1 (less than 1/3 of the skin area) or 2 (1/3 and more of the skin area), for each of the following five symptoms: redness/scratch marks, edema/lichenification/thickening, hemorrhage/scabbing, erosion, and desquamation.
Topical application of betamethasone and tetracycline to NC/Nga mice
After the skin severity score had reached approximately 8–11, corresponding to moderate AD-like skin inflammation, 0.1% betamethasone ointment and 3% tetracycline ointment were applied topically to the dorsal skin and the bilateral auricle skin (50 mg/body [=50 mg/10 cm2 skin]) in each group (6 mice per group) once per day, except Sunday and any public holiday. The design of the experimental schedule is summarized in Figure 1.
Histopathological observations
The dorsal skin was removed 31 days after assessment of skin severity, fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at a thickness of 5 μm. Tissue sections were then stained with hematoxylin-eosin (HE) and toluidine blue (TB), respectively, and observed microscopically.
Measurement of serum total IgE levels
Blood samples were collected from the heart 31 days after assessment of skin lesion severity. The total IgE concentration in the serum was measured by enzyme-linked immunosorbent assay (ELISA) using a mouse IgE ELISA kit (Cedarlane, ON, Canada).
Quantification of Th1 and Th2 cytokine production from T lymphocytes in lymph nodes
Auricular lymph node cells were harvested on the 31st day of skin severity assessment and adjusted to 1 × 106 cells/mL in RPMI 1640 medium with L-glutamine (Sigma-Aldrich, St. Louis, MO, United States) containing 10% fetal bovine serum (Sigma-Aldrich), 25 mM Hepes (Sigma-Aldrich), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco RBL, Grand Island, NY, United States). The cultures (0.2 mL/well) were incubated in 96-well culture plates (Nunc, Roskilde, Denmark) in the presence of Dynabeads Mouse.
T-Activator CD3/CD28 (Life Technologies, Oslo, Norway) at 37°C in a humidified atmosphere with 5% CO2. The culture supernatants were collected after incubation for 48 h, and the interferon (IFN)-γ and IL-4 concentrations were measured using ELISA kits for quantification of murine IFN-γ and IL-4, respectively (R&D Systems, Minneapolis, MN, United States).
Detection of S. aureus on skin
Bacterial isolates were obtained from each skin lesion by applying a “Film Stamp” for 10 s to the affected dorsal skin (8 cm2 area [=2 cm × 4 cm]) (9). After an overnight culture on tryptic soy agar plates at 37°C, the colonies grown were characterized by color and diameter, and colony numbers were expressed as colony-forming units (CFUs) per 8 cm2 skin area. Microscopic examination of Gram-stained colonies and the PS Latex (Eiken Chemical, Tokyo, Japan) slide agglutination test were also carried out to identify these organisms. S. aureus was finally identified from the reaction profile based on the 20 biochemical tests included in the API STPH system (Biomérieux, Marcy-I’Etoile, France).
Statistical analysis
The data were expressed as means (± standard error of the mean [SEM]), and differences between means were analyzed using one-way ANOVA, followed by Tukey-Kramer multiple comparison test. Differences at p < 0.05 were considered to be statistically significant.
Results
Therapeutic effects of topically applied betamethasone and tetracycline on AD-like skin lesions in NC/Nga mice
Assessment of skin lesion severity in NC/Nga mice sensitized with a mite antigen and medication with ointment was started 13 days after initial sensitization with the mite antigen (Figure 1). Before treatment, the sensitized mice had moderate AD-like skin lesions, and the clinical skin lesion severity in the mite antigen-sensitized control mice (none) increased gradually with each successive assessment (Figure 2). Topical application of vehicle alone did not affect the skin severity score in NC/Nga mice (data not shown), as had also been observed in our previous study (17). However, monotherapy with either betamethasone ointment or tetracycline ointment alone began to suppress the increase in the skin severity score from the fourth day of assessment, although no significant difference between the therapeutic effects of the two drugs was apparent. On the other hand, the therapeutic efficacy of betamethasone ointment and tetracycline ointment in combination became apparent after 18 days of assessment, and its efficacy significantly exceeded that of monotherapy with either ointment alone, persisting throughout the experimental period. All mice in the control group (none) on the 31st day of assessment had AD-like skin lesions comprising redness/scratch marks, edema/lichenification/thickening, hemorrhage/scabbing, erosion and desquamation, and the difference in efficacy between the monotherapy and the combination therapy was macroscopically evident (Figure 3).
FIGURE 2
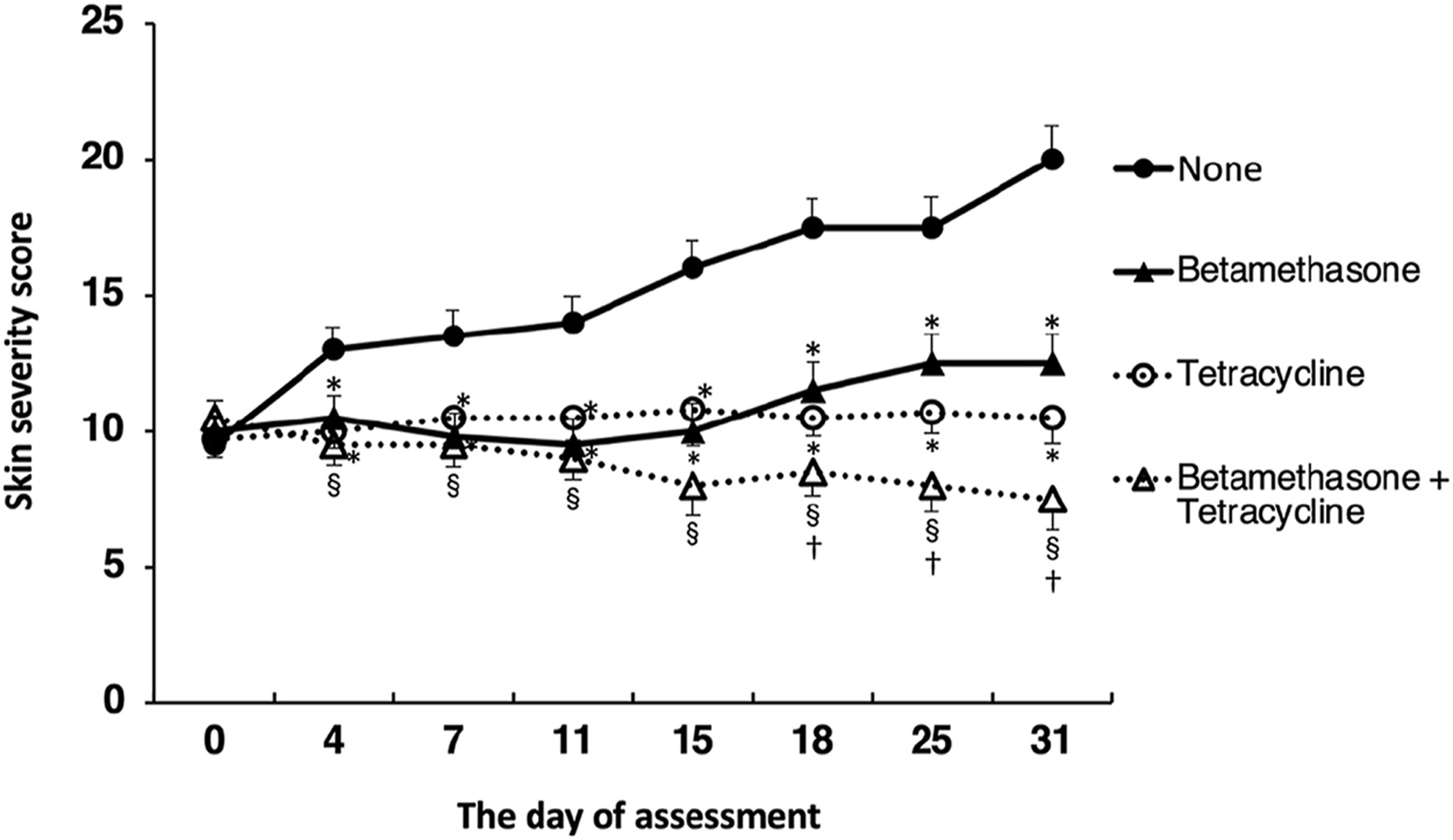
Effects of topically applied betamethasone and tetracycline on skin severity score. The results for each experimental group are expressed as means ± SEM (n = 6). *p < 0.01 versus none, §p < 0.01 versus none, †p < 0.05 versus betamethasone, tetracycline.
FIGURE 3
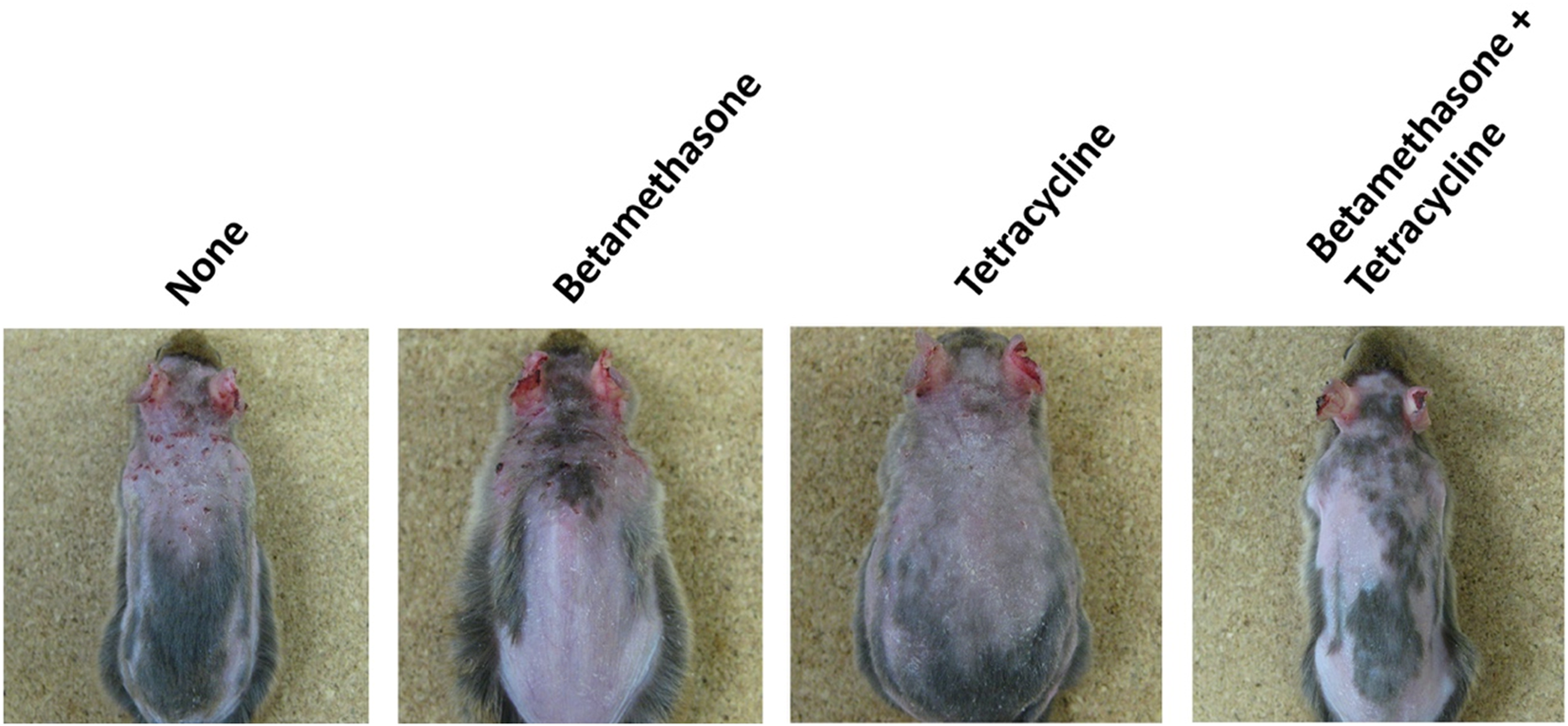
Macroscopic features of AD-like skin lesions in NC/Nga mice. The photograph shows skin lesions on the 31st day of assessment.
Effects of topical application of betamethasone and tetracycline on histopathological changes in dorsal skin
As shown in Figure 4, control mice (none) on the 31st day of skin lesion severity assessment showed epidermal hyperplasia and dense infiltration of inflammatory cells such as mast cells, eosinophils and lymphocytes, similar to the skin lesions of AD patients (Figure 4A). Topical application of betamethasone ointment alone or tetracycline ointment alone each had a slightly inhibitory effect on dermal infiltration of inflammatory cells. However, the level of this inhibition elicited by combined topical application of betamethasone ointment and tetracycline ointment was superior to that elicited by betamethasone alone or tetracycline alone. Specifically, the number of mast cells was markedly reduced by betamethasone and tetracycline in combination (Figure 4B). Similarly, the two ointments applied together also reduced the degree of epidermal hyperplasia.
FIGURE 4
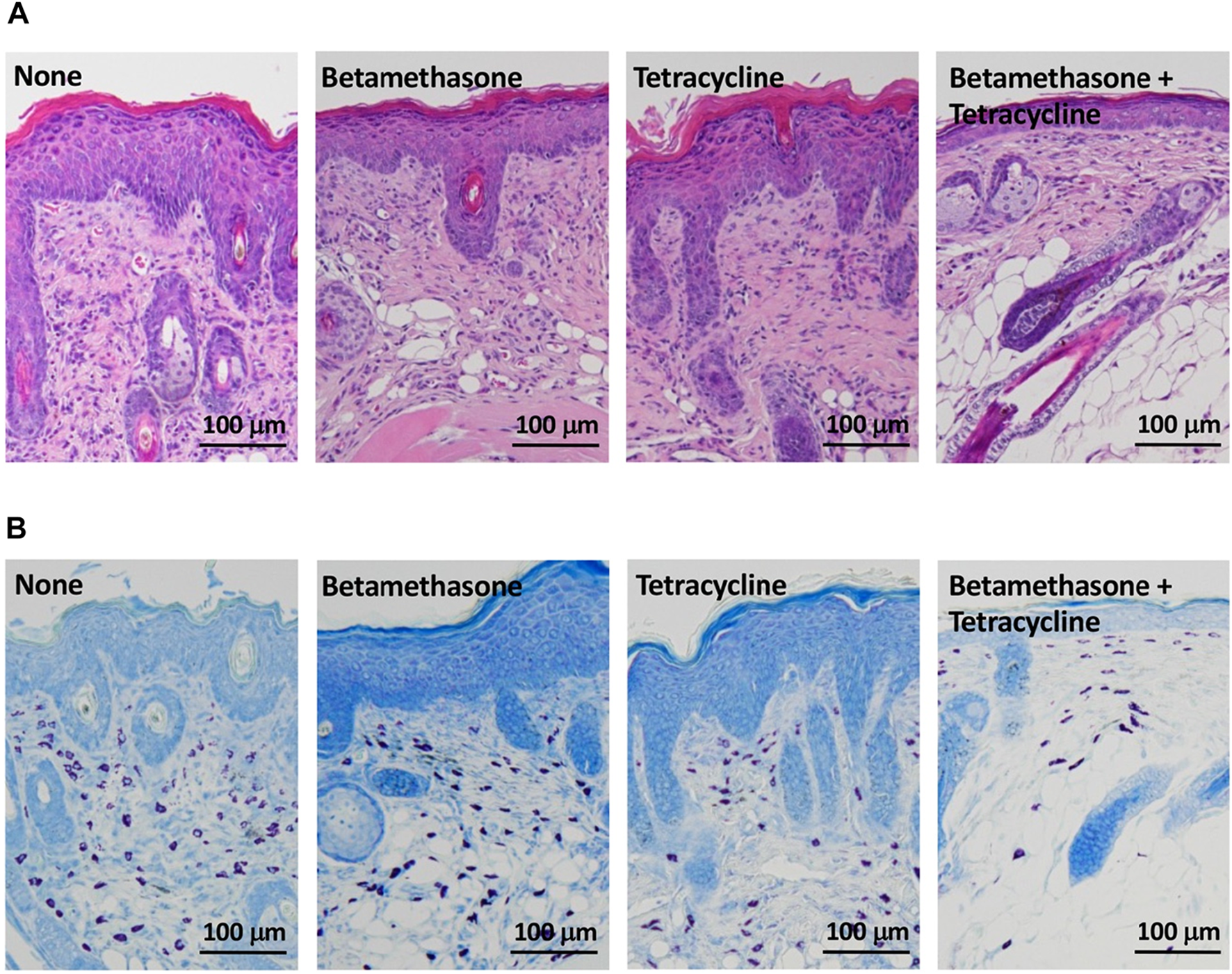
Histopathological analysis of AD-like skin lesions in NC/Nga mice. Skin sections were stained with hematoxylin-eosin (A) and toluidine blue (B) and observed at ×100. The photograph shows sections of skin lesions on the 31st day of assessment.
Effects of topically applied betamethasone and tetracycline on the serum total IgE level
The possible correlation between skin lesion severity and the serum IgE level was then investigated. The serum IgE level was elevated in mite antigen-sensitized NC/Nga mice, and topical application of vehicle alone did not affect it (data not shown). However, the serum total IgE level in control (untreated) mice on the 31st day of skin lesion severity assessment was significantly reduced to almost the same level by topical application of betamethasone alone or tetracycline alone, and combined application of betamethasone and tetracycline further reduced the IgE level relative to each agent used alone (Figure 5).
FIGURE 5
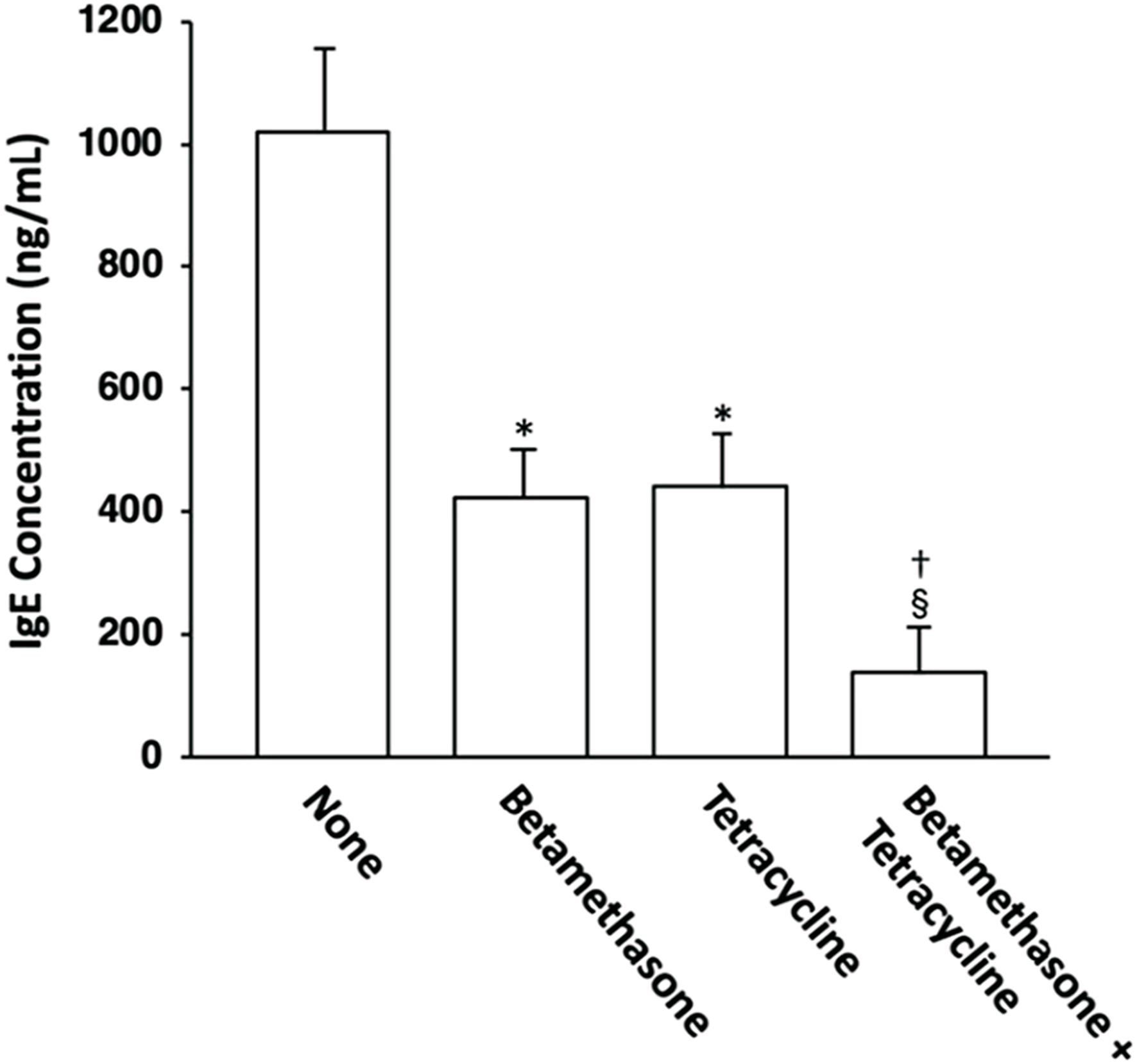
Effects of topically applied betamethasone and tetracycline on serum IgE concentration in NC/Nga mice with AD-like skin lesions. The serum total IgE levels on the 31st day of assessment were measured by ELISA. The results for each experimental group are expressed as means ± SEM (n = 6). *p < 0.01 versus none, §p < 0.01 versus none, †p < 0.01 versus betamethasone, tetracycline.
Effects of topical application of betamethasone and tetracycline on Th1 and Th2 cell development
The possible correlation between skin lesion severity and the level of Th1 and/or Th2 cell development was then examined. On the 31st day of skin severity assessment, ELISA for IFN-γ and IL-4 was carried out using culture supernatants of lymph node cells that had been stimulated via their cell surface CD3/CD28 molecules for 48 h in order to examine Th1 and Th2 cell development in auricular lymph nodes. As shown in Figure 6, elevated production of the Th1 cytokine IFN-γ and the Th2 cytokine IL-4, which serve as indices of Th1 and Th2 cell development, respectively, in lymph node cells from control (untreated) mice was significantly suppressed to almost the same level by topical treatment with betamethasone alone or tetracycline alone. However, topical application of vehicle alone did not affect the increased levels of Th1 and Th2 cell development observed in NC/Nga mice (data not shown), as had been seen in our previous study (17). In addition, the degree of inhibition of Th1 and Th2 cell development by combined application of betamethasone and tetracycline markedly exceeded that resulting from monotherapy with each drug.
FIGURE 6
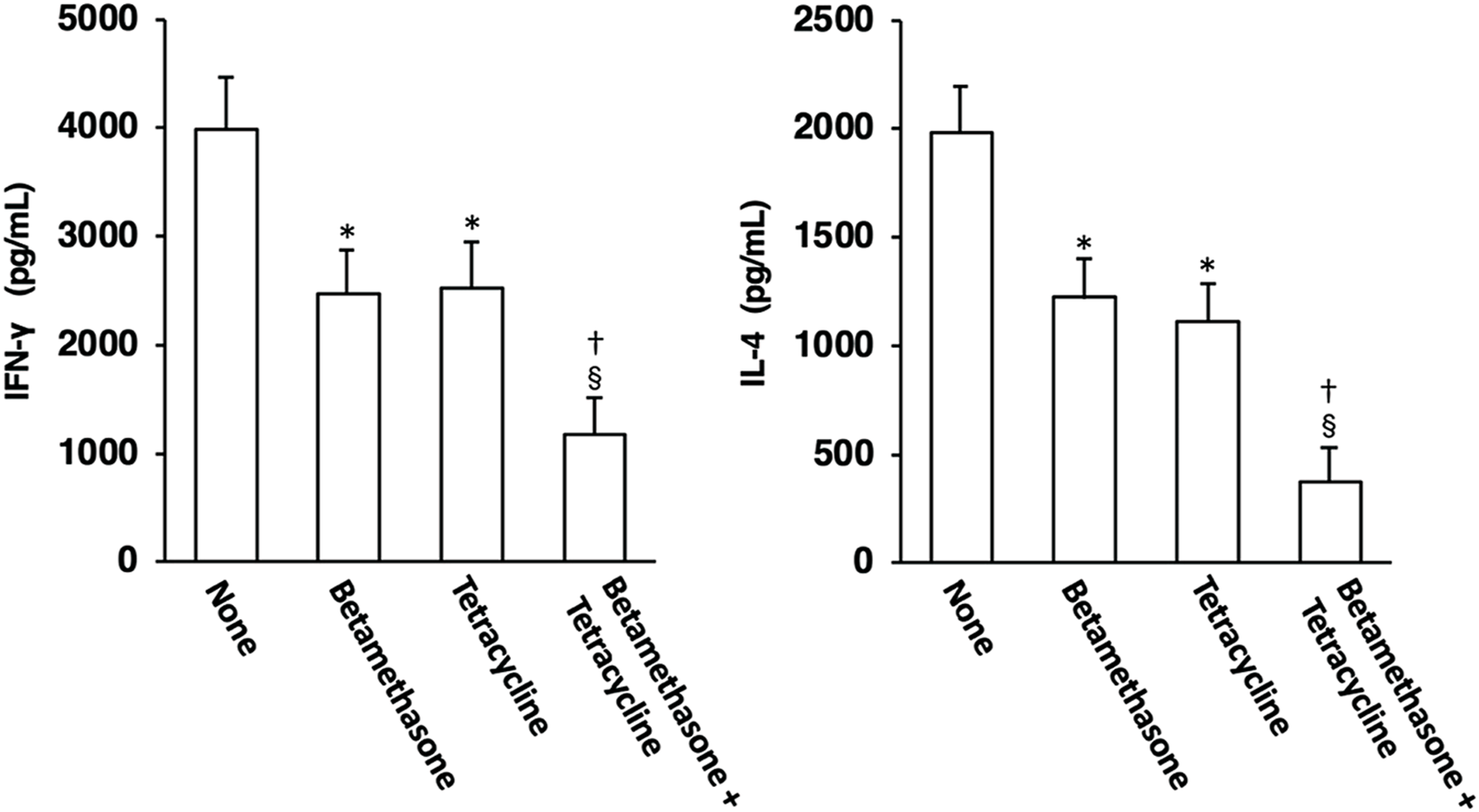
Effects of topically applied betamethasone and tetracycline on Th1 and Th2 cell development in auricular lymph nodes of NC/Nga mice with AD-like skin lesions. Auricular lymph node cells on the 31st day of skin severity assessment were stimulated through their surface CD3/CD28 molecules, and the concentrations of IFN-γ and IL-4 in the culture supernatants were determined by ELISA. Each culture was prepared in triplicate, and the mean value was obtained as a representative result for one experiment. The results are expressed as means ± SEM (n = 6). *p < 0.01 versus none, §p < 0.01 versus none, †p < 0.05 versus betamethasone, tetracycline.
Effect of topical application of betamethasone and tetracycline on skin colonization by S. aureus
The possible correlation between skin lesion severity and the degree of S. aureus skin colonization was then examined. The S. aureus CFU count per 8 cm2 area of dorsal skin in NC/Nga mice on the 31st day of skin severity assessment is shown in Figure 7. The lesioned skin of control (untreated) mice was heavily colonized by S. aureus. In comparison, the normal skin of NC/Nga mice was not colonized with this pathogen (data not shown). Topical application of betamethasone ointment to the lesioned skin decreased the S. aureus CFU values slightly.
FIGURE 7
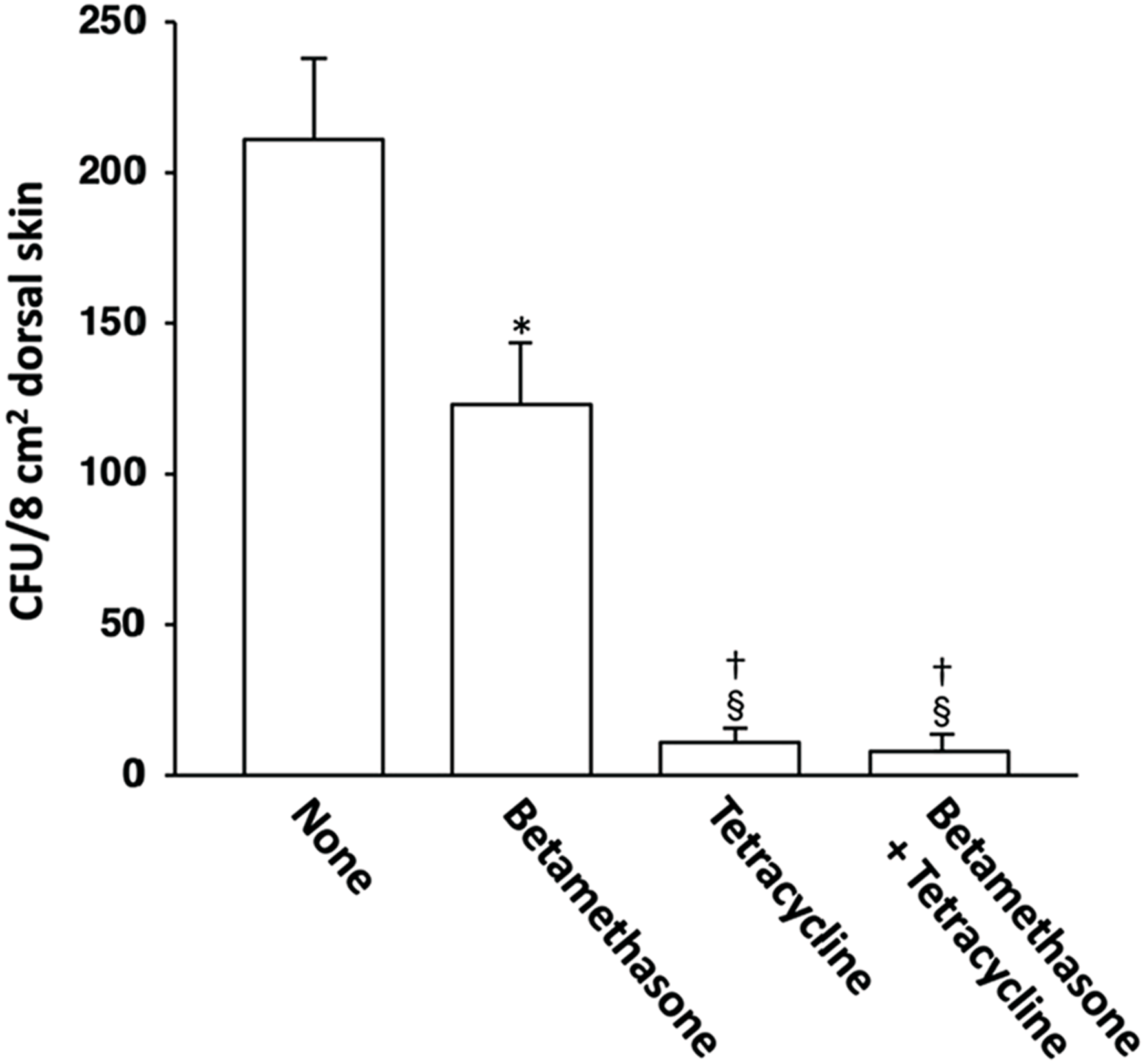
Effects of topically applied betamethasone and tetracycline on bacterial cell count of S. aureus on AD-like skin lesions in NC/Nga mice. The results for each experimental group are expressed as means ± SEM (n = 6). *p < 0.01 versus none, §p < 0.01 versus none, †p < 0.01 versus betamethasone.
However, when tetracycline was used alone or in combination with betamethasone, skin colonization by S. aureus was suppressed very strongly in comparison to treatment with betamethasone alone. We had already confirmed in our previous study that topical application of vehicle alone did not affect the degree of S. aureus colonization in NC/Nga mice (18).
Discussion
Th1/Th2 immune balance is closely connected with various immunological diseases, including allergy. It is well known that Th2 immunity is responsible for allergic immune responses and the subsequent pathogenesis of allergic diseases (19, 20, 21). AD is one such allergy-related disease, characterized by a marked increase in the count of Th2 cells in both peripheral blood and acute skin lesions (6). On this basis, it has been proposed that the Th2 immune response plays a key pathogenetic role in AD, and this is supported by the presence of blood eosinophilia and enhanced serum IgE levels in most AD patients (22, 23). Our previous study also suggested that the Th1 immune response also plays an instrumental role in the induction of AD-related chronic inflammation (15). Therefore, we have recently been focusing on an immunoregulatory approach for prevention of Th1 and Th2 cell development in AD patients.
Here we demonstrated that a combination of betamethasone and tetracycline ointment markedly suppressed the exacerbation of AD-like skin lesions in a NC/Nga mouse model, with a therapeutic efficacy superior to that of betamethasone alone or tetracycline alone. In comparison to the untreated group, skin lesion severity assessed macroscopically in terms of redness/scratch marks, edema/lichenification/thickening, hemorrhage/scabbing, erosion, and desquamation was significantly decreased by simultaneous application of 0.1% betamethasone ointment and 3% tetracycline ointment. These findings were also supported by histopathologic analysis. Combined topical application of betamethasone and tetracycline inhibited epidermal hyperplasia and dense infiltration of inflammatory cells such as mast cells, eosinophils, and lymphocytes more effectively than betamethasone alone or tetracycline alone. We had shown previously that LCs treated with betamethasone inhibited both Th1 cell and Th2 cell development in lymph nodes, and that LCs treated with tetracycline inhibited only Th2 cell development (15, 16). However, the present study showed that monotherapy with tetracycline also inhibited the development of Th1 cells in addition to Th2 cells, in a manner similar to betamethasone (16). This may have been due to the difference in the concentration of tetracycline between the two studies: in the previous study, 0.1% tetracycline had been used (15), whereas in the present study 3% tetracycline was used to ensure a better therapeutic effect. This suggested that the high concentration of tetracycline controlled not only Th2 cell but also Th1 cell development. The concentrations of the drugs in the ointments (0.1% betamethasone and 3% tetracycline) were equivalent to those used in clinical practice. Therefore, this type of dual therapy with betamethasone and tetracycline would be applicable to actual AD patients. Since it was considered that combined topical application of betamethasone and tetracycline to skin lesions of NC/Nga mice would target LCs in the epidermis, the affected LCs would then move to lymph nodes, where Th1 cell development and subsequent IFN-γ production, as well as Th2 cell development and subsequent IL-4 production, would be synergistically downregulated. This was appropriately reflected in the levels of Th1/Th2 cytokines in auricular lymph nodes on day 31 of skin severity assessment. It is known that the Th2 cytokine response is dominant in the acute phase of AD, and that in the late phase the Th1 cytokine response is also increased, contributing to chronic skin inflammation (6, 22, 24). These facts indicate that combined topical application of betamethasone and tetracycline can synergistically regulate both acute and chronic inflammation, thus contributing to amelioration of AD-like skin lesions in NC/Ng mice.
About 70%–80% of patients with AD show an increased serum level of IgE, which is associated with disease severity (6). An elevated serum IgE level was also observed in mite antigen-treated NC/Nga mice, and topical application of either betamethasone alone or tetracycline alone significantly reduced the serum IgE concentration. IL-4 receptor-mediated signaling in B cells is essential for induction of IgE synthesis (25). Therefore, the reduction in the serum IgE level in mite antigen-treated NC/Nga mice upon topical application of betamethasone or tetracycline could be explained by the degree of IL-4 expression in lymph nodes. Furthermore, topical application of both betamethasone and tetracycline synergistically reduced the serum IgE concentration and IL-4 expression level in NC/Nga mice. The difference of therapeutic efficacy between topical application of either betamethasone alone or tetracycline alone and that achieved by a combination of the two could be explained in terms of the serum IgE concentration, which precisely reflects the degree of Th2 immune response in the living body.
On the other hand, we have shown previously that S. aureus isolated from the lesional skin of AD patients is susceptible to tetracycline (15). Since in most AD patients the skin shows superficial S. aureus colonization and barrier disruption due to reduction of ceramide or filaggrin (26, 27), bacterial products such as staphylococcal enterotoxins, lipoteichoic acid and peptidoglycan would likely penetrate the skin and induce the production of Th2 cells, and in turn Th1 cells and related chemokines, leading to a Th2 immune response and a subsequent Th1 immune response, thus augmenting skin inflammation (6, 10, 11, 14, 28). Therefore, topical application of tetracycline to the skin lesions of AD patients appears to exert a marked effect involving a bactericidal action against S. aureus itself and inhibition of the Th1 and Th2 immune response through inhibition of the LC-mediated allergen-specific Th1 and Th2 cell development (15). Our recent study demonstrated heavy S. aureus colonization on the lesional skin of NC/Nga mice and showed that the count of this pathogen on the lesioned skin was correlated with lesion severity (18). Elimination of S. aureus colonization is important because otherwise it may trigger skin infection and subsequent impetigo. The results of the present study have shown that tetracycline alone acts against both S. aureus and dermatitis. Accordingly, topical administration of tetracycline would control both skin lesion severity and S. aureus colonization. Although treatment with betamethasone alone also decreased the S. aureus cell count on the lesional skin of NC/Nga mice, this reflected the steroidal effect of betamethasone, and not any intrinsic antibacterial activity. However, we confirmed that S. aureus colonization was eliminated more effectively by using an ointment containing both betamethasone with tetracycline than one containing betamethasone alone, which would be largely explainable by the antibacterial effect of tetracycline, resulting in a more marked decrease of the skin severity score than that achieved with betamethasone alone. This synergistic effect was also superior to treatment with tetracycline alone, which would have been attributable to not only the reduced lesional S. aureus count, but also the synergistic immunosuppressive effects of the two drugs. The standard treatment for AD is mainly topical steroids, and topical antibiotics are generally not actively applied for the disease unless impetigo is present in the skin lesions. However, the present observations suggest that in order to fully heal AD, both an antibacterial action against S. aureus on the lesional skin and inhibition of excessive Th1/Th2 cell development are necessary. Therefore, irrespective of the presence of impetigo, if skin lesions are colonized by S. aureus and are sensitive to tetracycline, aggressive use of the latter in topical form along with betamethasone ointment would be a more effective treatment strategy for AD.
Conclusion
Our results have demonstrated that combined topical application of betamethasone and tetracycline can synergistically inhibit the development of AD-like skin lesions and the Th1/Th2 immune response in NC/Nga mice through both immunomodulative and bactericidal actions. This dual therapy appears to have potential as a new strategy for AD patients with superficial S. aureus colonization.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Animal Care and Use Committee of Meiji Pharmaceutical University.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by JSPS KAKENHI Grant Number JP22K06779.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Weidinger S Beck LA Bieber T Kabashima K Irvine AD . Atopic dermatitis. Nat Rev Dis Primers (2018) 4:1–21. 10.1038/s41572-018-0001-z
2.
Chamlin SL Chren MM . Quality-of-life outcomes and measurement in childhood atopic dermatitis. Immunol Allergy Clin North America (2010) 30:281–8. 10.1016/j.iac.2010.05.004
3.
Deckers IAG McLean S Linssen S Mommers M van Schayck CP Sheikh A . Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: A systematic review of epidemiological studies. PLoS One (2012) 7:e39803. 10.1371/journal.pone.0039803
4.
Bieber T . Atopic dermatitis. N Engl J Med (2008) 358:1483–94. 10.1056/NEJMra074081
5.
Koh LF Ong RY Common JE . Skin microbiome of atopic dermatitis. Allergol Int (2022) 71:31–9. 10.1016/j.alit.2021.11.001
6.
Leung DY Boguniewicz M Howell MD Nomura I Hamid QA . New insights into atopic dermatitis. J Clin Invest (2004) 113:651–7. 10.1172/JCI21060
7.
Ong PY Leung DMY . Immune dysregulation in atopic dermatitis. Curr Allergy Asthma Rep (2006) 6:384–9. 10.1007/s11882-996-0008-5
8.
Guzik TJ Bzowska M Kasprowicz A Czerniawska-Mysik G Wójcik K Szmyd D et al Persistent skin colonization with Staphylococcus aureus in atopic dermatitis: Relationship to clinical and immunological parameters. Clin Exp Allergy (2005) 35:448–55. 10.1111/j.1365-2222.2005.02210.x
9.
Matsui K Nishikawa A Suto H Tsuboi R Ogawa H . Comparative study of Staphylococcus aureus isolated from lesional and non-lesional skin of atopic dermatitis. Microbiol Immunol (2000) 44:945–7. 10.1111/j.1348-0421.2000.tb02587.x
10.
Matsui K Nishikawa A NishikAwA A . Peptidoglycan from Staphylococcus aureus induces Th2 immune response in mice. J Investig Allergol Clin Immunol (2012) 22:80–6.
11.
Matsui K Nishikawa A . Lipoteichoic acid from Staphylococcus aureus induces Th2-prone dermatitis in mice sensitized percutaneously with an allergen. Clin Exp Allergy (2002) 32:783–8. 10.1046/j.1365-2222.2002.01357.x
12.
Matsui K Nishikawa A . Percutaneous application of peptidoglycan from Staphylococcus aureus induces an increase in mast cell numbers in the dermis of mice. Clin Exp Allergy (2005) 35:382–7. 10.1111/j.1365-2222.2005.02190.x
13.
Matsui K Wirotesangthong M Nishikawa A . Percutaneous application of peptidoglycan from Staphylococcus aureus induces eosinophil infiltration in mouse skin. Clin Exp Allergy (2007) 37:615–22. 10.1111/j.1365-2222.2007.02673.x
14.
Matsui K Nishikawa A NishikAwA A . Percutaneous application of peptidoglycan from Staphylococcus aureus induces infiltration of CCR4+ cells into mouse skin. J Investig Allergol Clin Immunol (2011) 21:354–62.
15.
Matsui K Nojima Y Kajiwara Y Busujima K Mori Y . Topical application of doxycycline inhibits Th2 cell development mediated by Langerhans cells and exerts a therapeutic effect on atopic dermatitis. J Pharm Pharm Sci (2020) 23:86–99. 10.18433/jpps30847
16.
Matsui K Tamai S Ikeda R . Betamethasone, but not tacrolimus, suppresses the development of Th2 cells mediated by Langerhans cell-like dendritic cells. Biol Pharm Bull (2016) 39:1220–3. 10.1248/bpb.b16-00075
17.
Matsui K Tachioka K Onodera K Ikeda R . Topical application of josamycin inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. J Pharm Pharm Sci (2017) 20:38–47. 10.18433/J3GW3D
18.
Matsui K Nakamura M Obana N . Effects of josamycin on scratching behavior in NC/Nga mice with atopic dermatitis-like skin lesions. Biol Pharm Bull (2021) 44:798–803. 10.1248/bpb.b20-00976
19.
Kay AB . Allergy and allergic diseases: First of two parts. N Engl J Med (2001) 344:30–7. 10.1056/NEJM200101043440106
20.
Herrick CA Bottomly K . To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol (2003) 3:405–12. 10.1038/nri1084
21.
Nguyen THT Casale TB . Immune modulation for treatment of allergic disease. Immunological Rev (2011) 242:258–71. 10.1111/j.1600-065X.2011.01034.x
22.
Grewe M Bruijnzeel-koomen CAFM Schöpf E Thepen T Langeveld-Wildschut AG Ruzicka T et al A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today (1998) 19:359–61. 10.1016/S0167-5699(98)01285-7
23.
Leung DY Bieber T . Atopic dermatitis. The Lancet (2003) 361:151–60. 10.1016/S0140-6736(03)12193-9
24.
Sabin BR Peters N Peters AT . Chapter 20: Atopic dermatitis. Allergy Asthma Proc (2012) 33(S1):S67–9. 10.2500/aap.2012.33.3553
25.
Wu LC Scheerens H . Targeting IgE production in mice and humans. Curr Opin Immunol (2014) 31:8–15. 10.1016/j.coi.2014.08.001
26.
Imokawa G Abe A Jin K Higaki Y Kawashima M Hidano A . Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin?J Invest Dermatol (1991) 96:523–6. 10.1111/1523-1747.ep12470233
27.
Brown SJ Irwin McLean W . One remarkable molecule: Filaggrin. J Invest Dermatol (2012) 132:751–62. 10.1038/jid.2011.393
28.
Matsui K Kanai S Ikuta M Horikawa S . Mast cells stimulated with peptidoglycan from Staphylococcus aureus augment the development of Th1 cells. J Pharm Pharm Sci (2018) 21:296–304. 10.18433/jpps29951
Summary
Keywords
atopic dermatitis, dual therapy, betamethasone, tetracycline, mouse model, Staphylococcus aureus , Langerhans cell
Citation
Matsui K, Kobayashi M, Nagano M and Matsuoka M (2023) Effects of dual therapy with betamethasone and tetracycline in a NC/Nga mouse model of atopic dermatitis. J. Pharm. Pharm. Sci 26:11182. doi: 10.3389/jpps.2023.11182
Received
09 November 2022
Accepted
10 January 2023
Published
19 January 2023
Volume
26 - 2023
Edited by
Fakhreddin Jamali, University of Alberta, Canada
Updates
Copyright
© 2023 Matsui, Kobayashi, Nagano and Matsuoka.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katsuhiko Matsui, kmatsui@my-pharm.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.