Dear Editors,
Epidermal nevi are hamartomas consisting of keratinocytes, melanocytes, sebaceous glands, hair follicles, apocrine and eccrine glands, and smooth-muscle cells. Epidermal nevus syndrome (ENS) is a multiorgan disease associated with epidermal nevi [1]. There are many variants of ENS such as Schimmelpenning syndrome, Becker nevus syndrome, and cutaneous skeletal hypophosphatemia syndrome [1]. Of these, cutaneous skeletal hypophosphatemia syndrome manifests as low serum levels of phosphorus, bone dysplasia, and notably increased serum levels of fibroblast growth factor 23 (FGF23) [2]. High FGF23 levels induce abnormally low phosphorus levels by both increasing phosphorus excretion in the urine and preventing absorption in the intestinal tract, and may lead to hypophosphatemic osteomalacia (HO) [2]. In this paper, we report a case of ENS with elevated FGF23 levels, which induced HO and difficulty in walking, that was successfully treated with burosumab, an anti-FGF23 antibody.
A 36-year-old man was admitted to our hospital with progressive difficulties in walking due to lower-back and left-leg pain that was so severe he had to use a wheelchair. The pain had started during his childhood, when he had developed mild difficulty in walking. He also presented with multiple brown plaques aligned with Blaschko’s lines and pigmented papules in a segmental pattern that extended from the left side of his head to his neck (Figures 1A, B). At the age of 12 years, the tumors were excised and pathological examination revealed papillated epidermal hyperplasia and hypermelanosis (Figure 1C). Nevus cells were identified in the dermis of the pigmented papules (Figure 1D), leading to a diagnosis of epidermal nevi. Other than the epidermal nevi, the examination of the patient was remarkable for the absence of dental abnormalities, café au lait spots, limb shortening, or relevant family history.
FIGURE 1
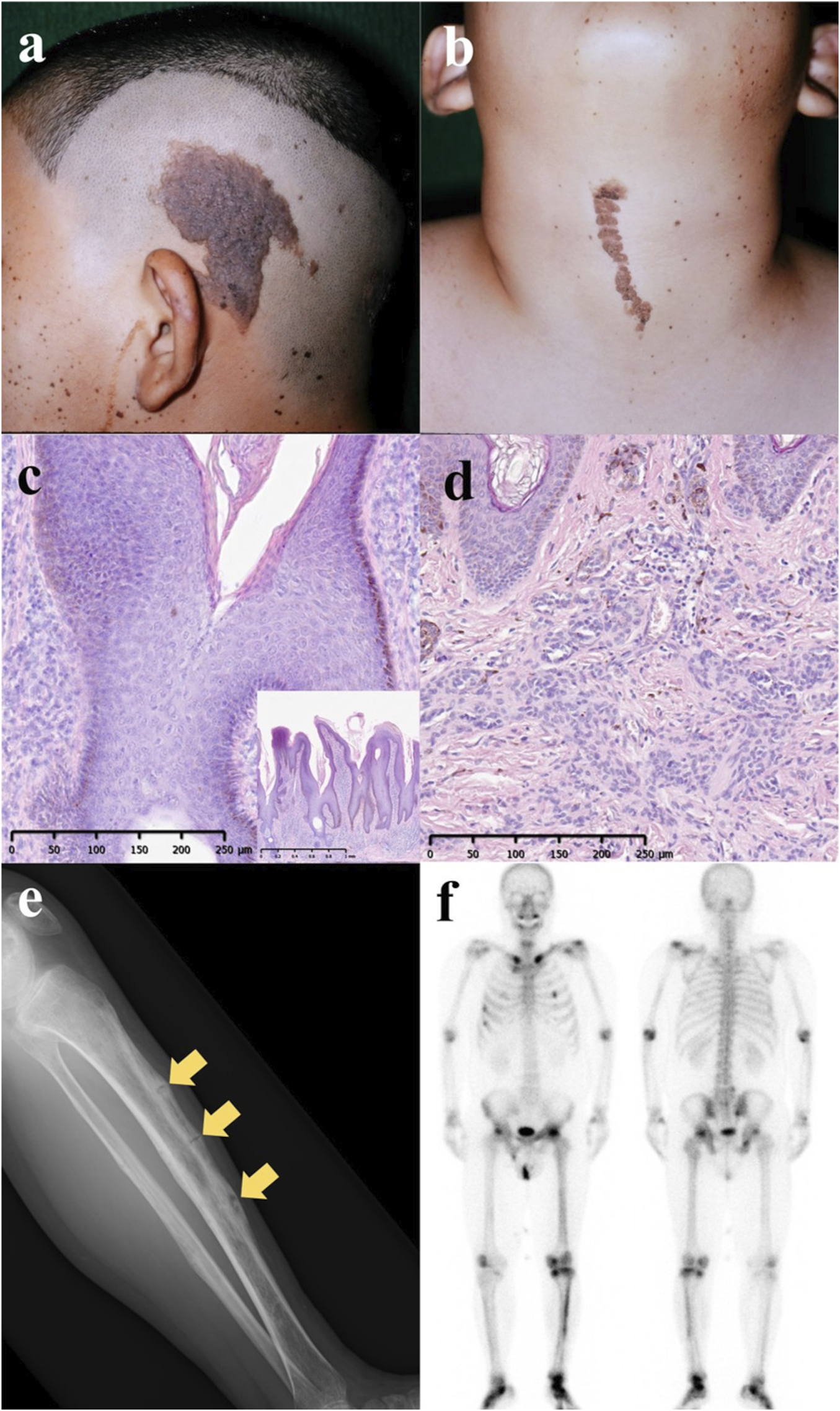
(A,B) Congenital brown plaques and pigmented papules located in the left temporal and anterior neck regions at 12 years of age. (C) Histopathological examination of the plaque revealed papillated epidermal hyperplasia and hypermelanosis (hematoxylin and eosin staining; scale bars: 250 μm and 1 mm). (D) Histopathological analysis of the pigmented papules indicated the presence of nevus cells within the dermis (hematoxylin and eosin staining; scale bars: 250 μm). (E) Radiographs of the left leg demonstrated osteomalacia with multiple pseudofractures (Looser zones, indicated by arrows). (F) Bone scintigraphy (99m Tc-MDP) showed increased uptake in the clavicles, ribs, pelvis, and lower extremities, and correlated with the location of pain in the left lower extremity.
X-ray imaging of his left leg revealed osteomalacia with multiple pseudofractures (Looser zones) (Figure 1E). His average hip-joint bone density was 64% of the value typical for young adults (right: 84%; left: 45%). Blood analysis revealed low levels of inorganic phosphorus (1.8 mg/dL; reference range: 2.2–5.3 mg/dL) and elevated alkaline phosphatase (274 U/L; reference range: 38–113 U/L), bone-specific alkaline phosphatase (55.6 μg/L; reference range: 3.7–20.9 μg/L), and FGF23 (113 pg/mL; reference range: 19.9–52.9 pg/mL). Bone scintigraphy (99m Tc-MDP) revealed increased uptake in the clavicle, ribs, pelvis, and lower extremities, especially on the left (Figure 1F). Somatostatin-receptor scintigraphy excluded tumor-induced osteomalacia.
Based on the available evidence, we diagnosed the patient as having ENS with FGF23-associated HO. The differential diagnosis for this condition includes X-linked hypophosphatemic (XLH) rickets with dental anomalies and ectopic calcification, McCune–Albright syndrome, and Jansen-type metaphyseal chondrodysplasia; however, we ruled out all of these conditions based on the patient’s clinical history.
We treated the patient with burosumab (1 mg/kg) to block FGF23 and improve his condition. His pain level on the Brief Pain Inventory-Short Form pain scale improved from 5 to 2, and as early as 5 weeks after the initiation of burosumab he was able to walk without assistance. The alkaline phosphatase level decreased to 187 U/L and that of inorganic phosphorus increased to 6.3 mg/dL. Following the initiation of burosumab therapy, radiographic evaluation of the patient’s left leg at 3 months showed bone union at the Looser zones, and after 1 year, the mean bone density of the hip joints increased to 76% (right: 89%; left: 63%) of the young-adult reference value. After confirming the improvement of both subjective symptoms and disease signs, we reduced the dose of burosumab from 1 mg to 0.5 mg at 42 weeks and to 0.25 mg at 58 weeks. We found no evidence of treatment-related adverse events.
The standard treatment for ENS with elevated FGF23 levels is oral phosphate and calcitriol [2, 3]. While this treatment may temporarily elevate the phosphorus level, it fails to fully restore the decreased phosphate reabsorption in the kidneys [2]. Thus, secondary or tertiary hyperparathyroidism and nephrocalcinosis frequently occur in these patients [2]. Moreover, the treatment has a limited effect on skeletal deformities and bone pain.
Burosumab is a humanized immunoglobulin G1 monoclonal antibody that binds to FGF23 and inhibits its function, leading to an increase in phosphorus levels. To the best of our knowledge, only four pediatric cases and one adult case of ENS treated with burosumab have been reported [2–5]. In those studies, burosumab doses of 0.3–1.4 mg/kg improved the phosphorus levels of all patients. Moreover, burosumab alleviated musculoskeletal pain, corrected skeletal anomalies, and enhanced physical functioning. Although one patient in one of those studies developed tertiary hyperparathyroidism, it was unclear whether this was an adverse effect of burosumab [4], as no other severe adverse events were reported.
Given that the previous studies used burosumab following conventional oral medication, ours is the first we know of in which burosumab was used as the first-line therapy. Although we have no direct evidence showing the advantages of using burosumab as first-line therapy, one study suggested that early use of burosumab in children might minimize skeletal deformities [2]. In our patient, early burosumab treatment improved multiple bone pseudofractures. Notably, unlike conventional ENS treatments, burosumab did not cause nephrocalcinosis.
We prescribed burosumab at 1 mg/kg (the recommended range for patients with XLH is 0.8–1.0 mg/kg). Although the patient’s symptoms (bone pain, difficulty in walking) rapidly improved, the phosphorus level exceeded the normal range. Thus, we reduced the dose gradually and found that 0.25 mg/kg was sufficient to keep the phosphorus level within a normal range while maintaining the symptomatic improvements. For our patient, a maintenance dose of less than 0.25 mg/kg may be appropriate.
In conclusion, dermatologists should consider ENS in the differential diagnosis of patients presenting with epidermal nevi and difficulty in walking. Diagnostic procedures, including radiographic imaging and laboratory data such as serum levels of phosphorus and alkaline phosphatase are essential. For patients exhibiting elevated FGF23 levels, burosumab may become a first-line treatment because it improves hypophosphatemia, skeletal deformities, and intense pain, seemingly without significant adverse effects.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was conducted in accordance with the local legislation and institutional requirements. The participant provided consent to partake in the study. Written informed consent was obtained from the participant for the publication of any potentially identifiable images or data included in this article.
Author contributions
SY, KY, TF, NK, and YF wrote the manuscript, and NK, JM, KS, and MM contributed interpretation of the studies. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Garcias-LadariaJCuadrado RosónMPascual-LópezM. Epidermal nevi and related syndromes - Part 2: nevi derived from adnexal structures. Actas Dermosifiliogr Engl Ed (2018) 109(8):687–98. 10.1016/j.ad.2018.05.004
2.
SugarmanJMaruriAHamiltonDJTabatabaiLLucaDCimmsTet alThe efficacy and safety of burosumab in two patients with cutaneous skeletal hypophosphatemia syndrome. Bone (2023) 166:116598. 10.1016/j.bone.2022.116598
3.
MerzLMBuergerFZiegelaschNZenkerMWielandILipekTet alA case report: first long-term treatment with burosumab in a patient with cutaneous-skeletal hypophosphatemia syndrome. Front Endocrinol (Lausanne) (2022) 13:866831. 10.3389/fendo.2022.866831
4.
HuynhCGillisAFazendinJAbdullatifH. A case report to assess the safety and efficacy of Burosumab, an investigational antibody to FGF23, in a single pediatric patient with Epidermal Nevus Syndrome and associated hypophosphatemic rickets. Bone Rep (2022) 17:101605. 10.1016/j.bonr.2022.101605
5.
KhadoraMMughalMZ. Burosumab treatment in a child with cutaneous skeletal hypophosphatemia syndrome: a case report. Bone Rep (2021) 15:101138. 10.1016/j.bonr.2021.101138
Summary
Keywords
burosumab, epidermal nevus syndrome, hypophosphatemic osteomalacia, FGF23, ENS
Citation
Yoshida S, Yatsuzuka K, Fujibuchi T, Nakaguchi H, Kohri N, Muto J, Shiraishi K, Murakami M and Fujisawa Y (2024) Adult epidermal nevus syndrome with hypophosphatemic osteomalacia treated with burosumab: a case study and literature review. J. Cutan. Immunol. Allergy 7:12575. doi: 10.3389/jcia.2024.12575
Received
17 December 2023
Accepted
07 February 2024
Published
16 February 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Yoshida, Yatsuzuka, Fujibuchi, Nakaguchi, Kohri, Muto, Shiraishi, Murakami and Fujisawa.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoshi Yoshida, ehimeehime101@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.