Abstract
Introduction:
Dystonia is characterized by dysfunctional movements and postures and current treatments aim to reduce unwanted muscle activity. Dystonia also encompasses non-motor symptoms which are becoming increasingly recognized as important contributors to quality of life. Less attention has been paid to treating these non-motor symptoms. This systematic review was undertaken to describe what is known regarding non-motor symptom treatment in dystonia.
Methods:
A systematic review was undertaken of the published literature from 2019 to 2025. Studies on dystonia that included description of non-motor symptoms and changes after treatment were included.
Results:
408 records were identified for review with 89 meeting inclusion and exclusion criteria for full review. 22 reports and 10 additional studies from review of references were included in the review. Treatments were stratified by type of treatment (e.g., surgical, non-invasive neurostimulation, and botulinum toxin injection) as well as by type of dystonia (e.g., generalized vs. focal vs. segmental). Response of non-motor symptoms to surgical treatment were mixed. Ablative therapy showed some improvements in non-motor symptoms but with the inherent risks of ablative procedures. Botulinum toxin consistently improved mood and pain across multiple dystonia populations.
Conclusion:
This review summarizes the current state of treatment effects of non-motor symptoms in dystonia. In most cases, the treatments were primarily aimed at motor symptoms but changes were sometimes seen in non-motor symptoms as well. Better detection and treatment of non-motor symptoms in dystonia are needed to wholly treat patients with dystonia.
Introduction
Dystonia, not unlike Parkinson’s disease, is a syndrome in which motor symptoms are often accompanied by non-motor symptoms (NMS). These NMS may exert a more significant impact on a patient’s quality of life (QoL) and influence their overall experience of both the dystonia and its associated treatments [1]. The NMS most commonly described include anxiety and depression, pain and sensory abnormalities, sleep disturbances, and cognitive changes. While NMS were historically felt to be secondary to motor symptoms, and which certainly can contribute to the experience of NMS, we now understand these symptoms to be an independent feature of disease, out of proportion to motor symptom severity, and sometimes predating the onset of motor symptoms [2, 3]. Although nonmotor symptoms are demonstrated to be independent of motor symptoms, they commonly impact upon each other, making it difficult to fully separate distinct mood disorders from pain, or fatigue from sleep dysfunction, for example [2–4]. NMS are common in dystonia across varying etiologies and affected body areas [5]. This widespread prevalence of NMS in dystonia may be explained by a unifying theory of network dysfunction as evidenced by imaging studies showing abnormal activity in brain structures involved in both motor and nonmotor networks [6–9].
Neuropsychiatric symptoms
The impact of neuropsychiatric symptoms on patients’ lives cannot be overstated - an illustrative example is social phobia and social anxiety. Social phobia is common in cervical dystonia (CD), attributable to a sense of body deformation and anticipation of negative perception by others related to the disease. This perception is out of proportion to motor severity, leading to self-stigmatization: a self-conscious anxiety in public spaces and social situations, increasing isolation and depression [10, 11]. There seems to be an overall hypervigilance, an erroneous assignment of salience to unimportant stimuli that is common in CD with NMS. There is known network dysfunction in dystonia involving salience network structures, like the amygdala, and structures interacting with the salience network, like the cerebellum [6, 12–14]. In fact, the cerebellum modulates input related to sensorimotor, salience, and default mode networks, implicating it across nonmotor symptoms like pain and sensation perception, depression and anxiety [12]. Indeed, resting-state functional neuroimaging studies have found abnormal activity in salience networks as directed by the cerebellum, correlating with symptoms of depression [12]. Social phobia illustrates the challenge in identifying which NMS are primary to the disease and which are a result of other NMS, or of the motor symptom impact of dystonia. For instance, higher rates of disordered eating and substance use disorder are found in patients with dystonia, suggesting a primary etiology [5, 11, 15]. However, as shown above, a symptom like social phobia could lead to isolation and subsequent depression and maladaptive coping strategies. Anxiety and depression are also commonly reported in writer’s cramp, laryngeal dystonia, oromandibular dystonia (OMD), and blepharospasm, and are unrelated to motor symptom severity, even predating the onset of motor symptoms again suggesting a primary role in the disorder [16–21]. 32.3% of patients with isolated dystonia report a history of suicidal behavior, a rate significantly higher than that of the general population, highlighting the severity of impact of these symptoms on patients’ wellbeing [22].
Sleep/wake symptoms
Fatigue and excessive daytime sleepiness (EDS) are prevalent and significantly impact the QoL of patients with dystonia, influencing their perceptions of the disease and satisfaction with treatment [4, 10, 16, 20, 23–25]. Compared to healthy controls, patients with dystonia exhibit reduced daytime activity, as measured by self-report and accelerometry data [26]. Sleep disturbances are present in up to 70% of patients with CD [10]. Across various dystonia subtypes, patients experience higher rates of insomnia, poorer sleep quality, reduced sleep duration, and lower sleep efficiency, as well as greater EDS, compared to healthy controls [5, 26]. Dysregulation of circadian rhythm networks, particularly cerebellar hyperactivity, has been proposed as a potential contributing factor to these sleep-wake disturbances [7].
Pain
Pain is a prevalent feature of dystonia and has a significant impact on health-related QoL [5, 27]. Patients with blepharospasm frequently experience eye pain and photophobia [17]. Similarly in CD, pain commonly reported and relates to complex mechanisms, including increased muscle contraction affecting muscle spindles and their associated pain receptors, alterations in peripheral sensitivity due to neuropeptide activity, abnormal processing of pain pathways, and impaired inhibitory signalling [28]. A systematic review has demonstrated that botulinum toxin (BoNT) treatment can significantly alleviate pain, as evidenced by improvements in the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) pain scores [28]. Although BoNT treatment generally provides effective pain control, pain may recur between injection cycles and its persistence is associated with lower patient satisfaction with treatment [29, 30]. Deep brain stimulation (DBS) has shown variable impact on pain outcomes in prior studies, with some research indicating no effect and other studies showing significant improvements in pain scores independent from motor symptom improvement, suggesting that pain may be a primary and distinct symptom of dystonia [28].
Changes in cognition
Altered cognitive function is one of the least understood NMS of dystonia, as clinical changes are subtle and nonprogressive, and are difficult to glean from common screening measures like the Montreal Cognitive Assessment (MoCA) or the Mini Mental Status Exam (MMSE) [31]. Social cognition has been a focal point of cognitive research in CD, with deficits in this domain having substantial implications for comorbid anxiety [32]. Patients with CD demonstrate a reduced ability to recognize emotional expressions in both auditory and visual tasks compared to controls in some studies [33, 34]. Additionally smaller studies have reported lower performance in spatial working memory, word retrieval, and working memory in individuals with CD [35].
Patients with myoclonus-dystonia and CD both demonstrate impairment in their explicit sense of agency, a cognitive function dependent upon one’s ability to predict their actions’ impact on the external world [36, 37]. This deficit may implicate the cerebellum, which plays a key role in predicting feedback from self-generated actions and may indirectly contribute to dysfunction in the sense of agency network [36–39]. Some small studies have found executive deficits and slowed cognitive speed in CD patients; specifically, deficits of inhibitory control seem to be a feature of CD, even when controlling for patients on benzodiazepines [40].
Systematic review
The purpose of this study is to provide a descriptive review of recent publications related to the treatment of NMS in dystonia published over the past 5 years, and to provide a comprehensive description of this literature. We aim to reveal the benefits and limitations of common dystonia treatment approaches on NMS, and to uncover areas requiring further study.
Methods
This systematic review focused on the treatment of NMS in dystonia. PubMed, Embase, Web of Science, and Cochrane databases were searched with keywords “dystonia” AND “non-motor” with a publication date limit of 5 years (2019–2024). PubMed search was performed initially, and then an expanded search including the other databases was performed. To ensure the relevance of the evidence base, we limited our systematic review to studies published within the past 5 years. This timeframe was selected to capture the most up-to-date research reflecting recent developments in the field. Recently, the dystonia literature has increasingly emphasized the significance of NMS and their impact on treatment outcomes. A 2018 systematic review evaluated NMS outcomes in DBS for dystonia [41]. Subsequent studies, including reports published in 2020 and 2023 have underscored the importance of assessing NMS in the context of patient-centered outcomes of BoNT injection [42, 43]. In 2023, a Dystonia Medical Research Foundation expert meeting advocated for the systematic evaluation of NMS, including the potential repurposing of existing therapies to address these outcomes in clinical trials [44]. Collectively, these developments support the appropriateness of a 5-year publication window to capture the most pertinent and forward-looking research in this evolving area. To capture the latest publications, weekly email alerts for new publications were reviewed up until the point of submission in June 2025. Studies that discussed NMS of various types were included for review of title and abstract, and of the full text if needed. Studies that measured treatment impacts on NMS were selected for full review. Relevant references within these papers meeting criteria were also reviewed. We excluded studies of dystonia secondary to brain injury, case reports, animal studies, and publications not available in the English language.
Results
408 records were identified for review after the removal of duplicates. 89 reports meeting inclusion and exclusion criteria were selected for full text review. Of these, 22 reports were ultimately included, as well as 10 additional studies from review of references (Figure 1). Here, we organize the results by treatment type, and then by dystonia body distribution.
FIGURE 1
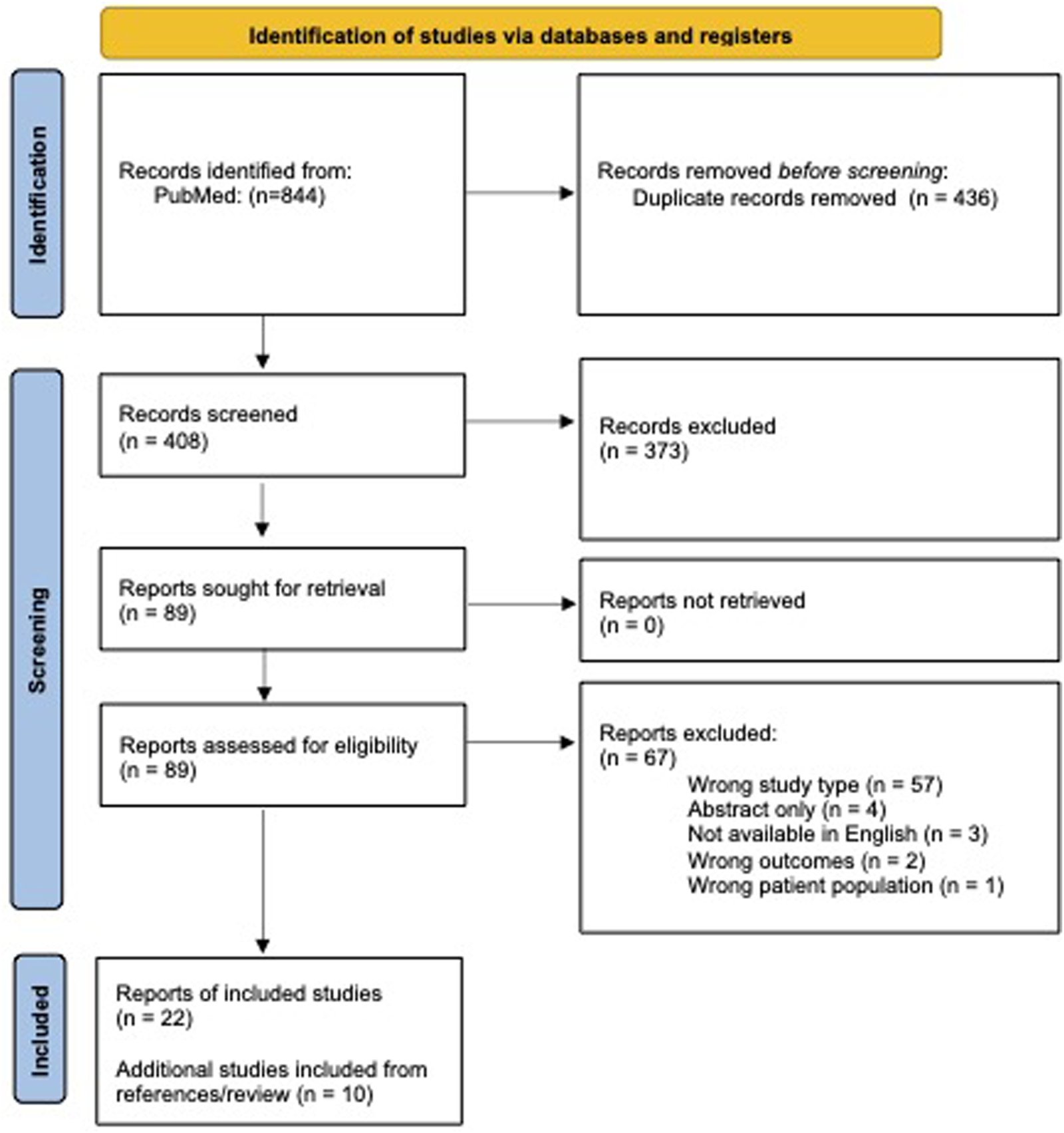
PRISMA 2020 flow diagram for systematic review [92].
Surgical interventions
Surgical interventions may be utilized in medication and toxin-refractory cases of dystonia. Various targets were investigated over the past 5 years including DBS of the globus pallidus internus (GPi), subthalamic nucleus (STN), and ventral intermediate nucleus of the thalamus (Vim); ablation of pallidothalamic tracts by focused ultrasound and radiofrequency; and cervical spinal cord stimulation. Motor symptoms improved with many of these interventions, however, the response of NMS was variable. There were 13 publications in the past 5 years (six case series; one randomized controlled trial [RCT]; five prospective, non-controlled studies; and one prospective, crossover, double-blind study) that reported on the impact of surgical interventions on NMS as secondary outcomes using a variety of scales [Table 1].
TABLE 1
| Study | Design | Intervention | Population | Sample size | Significant changes | NO significant changes | NMS scales |
|---|---|---|---|---|---|---|---|
| Honey et al. [45] | RCT | DBS Vim | Laryngeal dystonia | 6 | Mood | Cognition | BDI-II, MoCA |
| Khanom et al. [46] | Case series | DBS GPi | CD | 37 | Pain | Mood | TWSTRS-P, HADS-A, HADS-D |
| Horisawa et al [47] | Case series | RFA Pallidothalamic tract | CD | 35 | Pain | TWSTRS-P | |
| Horisawa et al [48] | Prospective, non-controlled study | FUS Pallidothalamic tract | CD | 10 | Mood | BDI, BAI, AES | |
| Shimizu et al [49] | Case series | Spinal cord burst stimulation | CD | 4 | Pain | VAS, MPQ2 | |
| Hao et al [50] | Prospective, non-controlled study | DBS STN | Meige syndrome | 30 | Mood, Cognition, Sleep | SDS, SAS, MoCA, DST, BNT, SDMT, PSQI | |
| Hao et al [51] | Prospective, non-controlled study | DBS GPi vs. DBS STN vs. Pallidotomy | Meige syndrome | 98 | Cognition, Depression, Sleep quality | MoCA, BDI, PSQI | |
| Liu et al [52] | Case series | DBS STN vs. GPi | Meige syndrome | 42 | Mood in STN group | Mood in GPi group, Sleep quality | HAMD, HAMA; PSQI |
| Krause et al [53] | Case series | DBS GPi or ViM | Myoclonus-dystonia | 7 | Depression | BDI | |
| Listik et al Front Neurol [54] | Prospective, non-controlled study | DBS GPi, STN, and/or STN-SN | Generalized dystonia (idiopathic or genetic) | 11 | Anxiety, pain | HADS-A, BPI, NPSI, MPQ | |
| Listik et al Eur J Pain [55] | Prospective, crossover, double-blind study | DBS GPI | Generalized dystonia (idiopathic or genetic) | 16 | Pain and sensory thresholds | See source paper | |
| Stavrinou et al [56] | Prospective, non-controlled study | DBS GPi | Any primary dystonia | 10 | Executive function | Mood All other cognitive measures |
COWAT, Stroop test, BDI, STPI, Other neuropsychological tests (see source paper) |
| Lin et al [57] | Case series | DBS GPi vs. STN | Isolated dystonia | 71 | Mood, Pain | Cognition | HAMA, HAMD, SF-36; MMSE, MoCA |
DBS study results.
Summary of Deep Brain Stimulation (DBS) study outcomes and scales used. Abbreviations: Ventral intermediate nucleus of the thalamus (Vim), cervical dystonia (CD), globus pallidus internus (GPi), subthalamic nucleus (STN), substantia nigra (SN), radiofrequency ablation (RFA), focused ultrasound (FUS). Beck depression inventory and second revision (BDI, BDI-II), Beck anxiety inventory (BAI), Apathy evaluating scale (AES), Hospital Anxiety and Depression Scale (HADS-A, HADS-D), Toronto Western Spasmodic Torticollis Rating Scale - Pain subscale (TWSTRS-P), Visual analog scale (VAS), Short-Form McGill Pain Questionnaire and second revision (MPQ, MPQ2), Zung self-rating depression and anxiety scales (SDS, SAS), Hamilton anxiety and depression scales (HAMA, HAMD), Montreal cognitive assessment (MoCA), Digit span test (DST), Boston naming test (BNT), Symbol digits modalities test (SDMT), Pittsburgh sleep quality index (PSQI), Brief pain inventory (BPI), Neuropathic pain symptom inventory (NPSI), Controlled Oral Word Association Test (COWAT), State-Trait Personality Inventory (STPI), 36-Item Short Form Survey (SF-36).
Focal dystonia
In a randomized, double-blind crossover trial of six patients with laryngeal dystonia, VIM-DBS improved mood as assessed with the Beck Depression Inventory (BDI-II). Cognition as measured by Montreal Cognitive Assessment (MoCA) was not impacted [45].
GPI-DBS was found to significantly improve pain as measured after 5 years of stimulation by TWSTRS-Pain subscale (TWSTRS-P) in a study of 37 patients with CD. However, this study did not find an impact of GPI-DBS on mood as measured by the Hospital Anxiety and Depression Scale (HADS) despite significant motor improvement [46].
Horisawa et al. evaluated the use of pallidothalamic tractotomy in patients with CD [47, 48]. In 2022, the group reported on 35 patients who underwent unilateral radiofrequency ablation of Forel’s Field H1. Pain scores improved by 40% as measured by TWSTRS pain subscale (p = 0.0029). This study reported a high incidence of adverse events in 50% of patients [47]. Expanding upon this work, the group reported on ten patients with CD in 2024 who underwent focused ultrasound pallidothalamic tractotomy. Participants demonstrated modest improvement at 6 months in depression, anxiety, and apathy as assessed by BDI, BAI, and apathy evaluating scales. Adverse events included prolonged loss of hand dexterity in 30%, thought to be related to associated hypotonia [48]. Both studies were limited by short term follow up ranging from 6–18 months, leaving the important question of the long-term benefits of ablative surgeries to future study.
Cervical spinal cord tonic stimulation has demonstrated temporary motor improvements in patients with CD in a double-blind, crossover trial, though the observed effects may have been attributable to a placebo response associated with the paresthesia induced by tonic stimulation [58]. In a recent open-label pilot study by Shimizu et al. (2020), burst stimulation, which does not induce paresthesia, was evaluated in four patients with CD. In follow-up assessments ranging from six to 42 months, participants demonstrated a 50% improvement in TWSTRS scores, an 82% improvement in the Burke-Fahn-Marsden Dystonia Rating Scale, and up to a 97% reduction in pain, as measured by the visual analog scale (VAS) and Short Form McGill Pain Questionnaire 2 (SF-MPQ-2) [49]. While the study is limited by small sample size, study design, and the relatively short follow-up period, these promising results suggest this treatment modality warrants further investigation, particularly for patients with refractory pain despite optimal medication and BoNT treatment.
Segmental dystonia
In the past 5 years, three large case series examined the effects of DBS in patients with Meige syndrome (n = 170) [50–52]. Mood outcomes were assessed using the BDI, Zung Self-rating Depression and Anxiety Scales (SDS and SAS), as well as the Hamilton Anxiety and Depression Rating Scales (HAMA and HAMD). Hao et al. (2023) reported on 30 patients with Meige syndrome who underwent STN-DBS, finding no significant improvement in mood as measured by the SDS and SAS after 3 years of follow up [50]. Conversely, Liu et al. (2021) compared outcomes in 21 patients with Meige syndrome treated with STN-DBS to 21 patients treated with GPi-DBS. After 1 year of stimulation, the STN group showed significant improvements in mood, with 36.5% and 47.1% reductions in HAMD and HAMA scores, respectively (p = 0.014, p < 0.001), while the GPi group showed no significant changes [52]. No changes in sleep quality as measured by the Pittsburgh Sleep Quality Index (PSQI) were found. Notably, psychiatric symptoms were not correlated with motor severity in Meige syndrome.
Cognitive function was not significantly impacted by STN-DBS at 3 years of stimulation in 30 patients with Meige syndrome, as assessed by the MoCA, Boston Naming Test (BNT), Digit span test (DST), and Symbol Digit Modalities Test (SDMT) [50]. Sleep disorders were observed in 23% of participants, typically developing three to 15 years before the onset of motor symptoms, highlighting sleep dysfunction as a primary NMS of Meige syndrome [50]. However, neither GPi-DBS (n = 21) nor STN-DBS (n = 51) led to significant improvements in sleep quality, as measured by the PSQI [50, 52].
Hao et al. (2025) expanded on this work in a larger study of the impact of GPi-DBS, STN-DBS, and pallidotomy on NMS in 98 patients with Meige syndrome at 3 years from intervention, finding no significant changes in cognitive function, depression, nor sleep quality as measured by MoCA, BDI, and PSQI; and no between-group differences [51].
Generalized dystonia
In an update of seven patients with myoclonus-dystonia undergoing GPi or Vim-DBS, depression symptoms as measured by the BDI were unaffected after 20 years of stimulation, despite notable improvements in myoclonus, dystonia, and disability scores [53].
A study of 11 patients with generalized dystonia (idiopathic or genetic, including four with DYT-THAP1 and two with DYT-PRKRA) found significant improvements in anxiety and pain following DBS in GPI, STN, and/or STN-SN, as measured by the HADS-A, Brief Pain Inventory (BPI), Neuropathic Pain Symptom Inventory (NPSI), and McGill Pain Questionnaire (MPQ) (p < 0.05, p = 0.043, p = 0.028, p = 0.028 respectively) [54].
Additionally, a study by the same group on 16 patients with idiopathic dystonia undergoing GPi-DBS assessed sensory changes and responses to DBS under ON and OFF conditions [55]. The study found that patients with dystonia had altered baseline sensory and pain thresholds in both dystonia and unaffected body parts, compared to healthy controls. DBS did not significantly affect pain or sensory thresholds in unaffected body areas, nor did it improve inhibitory modulation of pain signaling. The authors concluded that both motor and NMS in dystonia relate to spatial discrimination network dysfunction in motor, sensory, cognitive, and limbic networks [55].
Isolated dystonia of mixed body distribution
While most studies focus on specific dystonia populations categorized by body distribution (e.g., generalized, segmental, or focal dystonia), a few studies have reported the impact of DBS on NMS in heterogeneous populations. One such study of GPi-DBS in ten patients with generalized, segmental, or focal dystonia found no significant changes in mood after 12 months of stimulation, as measured by the BDI and State-Trait Personality Inventory (STPI) [56]. However, this study reported significant improvements in executive function as measured by the Controlled Oral Word Association Test (COWAT), Stroop test, and a trend toward improved performance on the Trail Making Test Part A (p = 0.43, 0.5, 0.51 respectively); although these results were seen in subsections of these tests, and within a small sample size, limiting the generalizability of these findings.
Lin S et al. (2023) compared the effects of GPi versus STN-DBS in 71 patients with isolated dystonia (25 generalized, 25 segmental, 10 focal, and 11 multifocal). Both targets produced equivalent long-term benefits for mood and pain, as measured by HAMA, HAMD, and the 36-item Short Form Survey [57]. However, the STN group experienced improvements in motor and mood symptoms within 1 month, while similar benefits in the GPi group were not observed until 6 months. Notably, neither DBS target had an impact on cognitive function, as assessed by the MMSE and MoCA. The authors concluded that STN-DBS may be preferred when rapid improvements in motor and mood symptoms are desired.
Botulinum toxin (BoNT) treatment
Eleven publications (one RCT, eight prospective non-controlled studies, one prospective controlled study, and one retrospective case-control study) investigating the response of NMS to BoNT injection were reviewed, with most studies focusing on CD populations [Table 2].
TABLE 2
| Study | Design | Intervention | Population | Sample size | Significant changes | NO significant changes | NMS scales |
|---|---|---|---|---|---|---|---|
| Gilman Kuric et al [59] | Prospective, non-controlled study | BoNT | CD | 30 | Mood, Pain, Cognition | BAI, BDI-II; TWSTRS-P, MoCA | |
| Costanzo et al [60] | Prospective, non-controlled study | BoNT | CD | 45 | Mood, Pain | Sleep quality | HAMA, HAMD, TWSTRS-Psy; TWSTRS-P, PSQI, ESS |
| Sugar et al [61] | Prospective, non-controlled study | BoNT | CD | 60 | Mood | STAI | |
| Moriarty et al [62] | Prospective, non-controlled study | BoNT | CD | 53 | Mood, Pain | BDI-II, BAI, TWSTRS2-P | |
| Elshebawy et al [63] | Prospective, non-controlled study | BoNT | CD | 16 | Depression, Pain, Sleep quality | Sleep efficiency | PSQI, BDI, MPQ |
| Marciniec et al [64] | Retrospective case-control study | BoNT | CD | 50 | Pain | TWSTRS-P, PNRS | |
| Kongsaengdao et al [65] | RCT | BoNT | CD | 52 | Depression | PHQ-9, CES-D | |
| Marfoli et al [66] | Prospective, controlled study | BoNT | Focal dystonias | 11 | Rumination about body defects | Distress, body image satisfaction | VAS |
| Gupta et al [67] | Prospective, non-controlled study | BoNT | Focal dystonias | 65 | Depression, Anxiety | BDI, HAMA | |
| Yoshida et al [68] | Prospective, non-controlled study | BoNT | OMD | 408 | Pain, Sleep, Mood | OMDQ-25 | |
| Zheng et al [69] | Prospective, non-controlled study | BoNT-A | Meige syndrome | 75 | Mood | Sleep | HAMA, HAMD; PSQI, ISI, ESS |
BoNT study results.
Summary of Botulinum Toxin Injection (BoNT) study outcomes and scales used. Abbreviations: Cervical Dystonia (CD), Beck anxiety inventory (BAI), Beck depression inventory and second revision (BDI, BDI-II), Toronto Western Spasmodic Torticollis Rating Scale - Pain subscale and Psych subscale (TWSTRS-P, TWSTRS-Psy), Montreal cognitive assessment (MoCA), Hamilton anxiety and depression scales (HAMA, HAMD), Pittsburgh sleep quality index (PSQI), Epworth Sleepiness Scale (ESS), State-Trait Anxiety Inventory (STAI), Pain Numeric Rating Scale (PNRS), Patient Health Questionnaire-9 (PHQ-9), Epidemiological Studies-Depression Scale (CES-D),Visual analog scale (VAS), Oromandibular Dystonia Questionnaire (OMDQ-25), Insomnia Severity Index (ISI).
Focal dystonia
Over the past 5 years, six studies have examined the impact of BoNT injection on mood symptoms in CD. Four studies found improvements in mood at the peak of BoNT effect (four to 6 weeks post-injection) in a combined total of 121 patients. These improvements were measured using various scales, including the BAI, BDI-II, HAMA, HAMD, State-Trait Anxiety Inventory (STAI), and TWSTRS-Psych [59–61, 63]. In contrast, two studies with follow up intervals ranging from 3 months to 2 years reported no sustained impact on mood symptoms (n = 105), as measured by BDI-II, BAI, Patient Health Questionnaire-9 (PHQ-9), and Center for Epidemiologic Studies Depression Scale (CES-D) [62, 65]. These findings suggest that BoNT has an immediate impact on mood symptoms, but may not produce sustained modifications of mood symptoms.
Pain consistently responded to BoNT across four studies involving 141 patients with CD [59, 60, 63, 64]. Pain was measured using the MPQ, TWSTRS-Pain and Pain Numeric Rating Scale (PNRS). While BoNT treatment relieved pain in all studies, there were mixed findings regarding the duration of effect. Marciniec et al in a study of 50 patients with CD found pain reduction with BoNT treatment lasted longer than motor symptom improvement [64]. However, other studies reported that pain tended to reappear between injection cycles, contributing to patient dissatisfaction with BoNT treatment [29, 30]. These findings underscore the importance of regularly assessing pain during BoNT treatment using standardized scales to optimize patient wellbeing and treatment satisfaction.
Research on the impact of BoNT on cognitive function and sleep quality is limited. Only one study investigated cognitive function, with Gilman Kuric et al. (2024) reporting improvements in memory-guided saccades in 30 patients with CD following BoNT injection. While significant improvements were observed in memory, verbal fluency, and language ability, the median score change in each domain was only 0.5 to 1 point on the MoCA, raising questions about the clinical significance of these findings [59]. Regarding sleep, no improvements were found in sleep quality at 4 weeks post-injection, as measured by PSQI and ESS, in a study of 45 patients with CD [60]. However, sleep quality measures with the exception of sleep efficiency improved in another study of 16 patients with CD at 4 weeks from BoNT injection as measured by PSQI [63]. Notably, changes in NMS including mood, pain, and sleep did not correlate with changes in motor severity, supporting NMS as primary features of CD independent of motor symptom severity, and suggesting a direct effect of BoNT on these NMS [59–61].
In a study by Marfoli et al. (2024) on 11 patients with various focal dystonias, BoNT injection at 4 weeks resulted in improvements in depression and rumination about body defects, but there were no improvement in disease-related distress or body image satisfaction, as measured by the VAS [66]. In a study by Gupta et al. (2025) on 65 patients with various focal dystonias, BoNT injection resulted in no improvements in mood by BDI and HAMA scores at one and at 3 months [67].
A large case series of 408 patients with OMD treated with BoNT injections found significant improvements in NMS, including pain, sleep, mood and psychosocial functioning, as assessed by the Oromandibular Dystonia Questionnaire-25 (p < 0.001). Among these participants, 41% had tardive dystonia, however they were analyzed separately from idiopathic cases. Idiopathic patients demonstrated greater improvements in NMS compared to those with tardive dystonia (p < 0.001) [68].
Segmental dystonia
Zheng et al. (2023) investigated the effects of onabotulium toxin A in 75 patients with Meige syndrome, reporting significant improvements in anxiety and depression at one and 3 months post-injection, as measured by HAMA and HAMD. However, there were no improvements in sleep quality, as measured by PSQI, Insomnia Severity Index (ISI), and ESS. Additionally, the severity of NMS in Meige syndrome did not correlate with motor symptom severity [69].
Noninvasive therapies
Eight papers were published investigating physical therapy and psychotherapy approaches to treating dystonia and its NMS. Of these, three were RCTs, one was an open-label, proof-of-concept study (Table 3), and three were descriptive analyses based on interviews with patients and healthcare professionals.
TABLE 3
| Study | Design | Intervention | Population | Sample size | Significant changes | NO significant changes | NMS scales |
|---|---|---|---|---|---|---|---|
| Xu et al [72] | Prospective, non-controlled study | Vibro-tactile stimulation | CD | 44 | Pain | PPS | |
| Dec-Ćwiek et al [71] | RCT | KinesioTaping | CD | 17 | Mood, Sleep quality, NMS burden | HADS, PSQI, DNMSQuest | |
| van den Dool et al [70] | RCT | Specialized PT | CD | 72 | Pain, mood | TWSTRS-P, NRS, BAI, BDI | |
| Wadon et al [74] | RCT | Internet-based CBT | 20 | CD | Mood | BDI, HAMD, HAMA, GAD-7 |
Noninvasive, nonpharmacologic study results.
Noninvasive, nonpharmacologic study outcomes and scales used. Abbreviations: Cervical Dystonia (CD), Perceived Pain Scale (PPS), Hospital Anxiety Depression Scale (HADS), Pittsburgh sleep quality index (PSQI), Dystonia Non-Motor Symptoms Questionnaire (DNMSQuest), Physical therapy (PT),Toronto Western Spasmodic Torticollis Rating Scale - Pain subscale (TWSTRS-P), Beck anxiety inventory (BAI), Beck depression inventory (BDI), Cognitive behavioral therapy (CBT), Hamilton anxiety and depression scales (HAMA, HAMD), Generalized Anxiety Disorder-7 (GAD-7).
In one RCT, 40 patients with CD received specialized physical therapy, while 32 patients followed a standard physical therapy protocol. Both groups showed significant improvements in pain and mood as measured by TWSTRS pain scale, PRNS, BAI, and BDI. However, no between-group differences were observed at 12-month follow up [70]. Another negative crossover RCT, involving 17 patients with CD, compared BoNT treatment alone, BoNT with kinesiotaping, and BoNT with sham taping. No differences were found between groups in NMS, as measured by the Dystonia Non-Motor Symptoms Questionnaire, HADS and PSQI [71]. Xu et al. (2024) conducted a proof-of-concept study on the effects of vibrotactile stimulation on pain management in 44 patients with CD. While modest improvements in pain were observed, the study lacked a control group, limiting the interpretation of results [72]. McCambridge et al. (2019) published a descriptive analysis of physical activity in 263 patients with dystonia, including 40% with CD. The majority of participants reported worsening of motor and NMS, such as pain and fatigue, with high-impact physical activities (e.g., running, jogging, and brisk walking), while low-impact exercise (e.g., light walking, general stretching, yoga, or pilates) were less likely to exacerbate symptoms [73].
Four papers focused on psychotherapeutic or behavioral interventions for managing NMS in dystonia. Wadon et al. (2021) investigated an internet-based cognitive behavioral therapy (CBT) program as compared to routine care in a small, randomized controlled study of 20 patients with CD over a 6-month period. Although participants in the intervention group showed trends toward improved anxiety and depression scores, and reported that the treatment was beneficial, no significant differences were observed between the two groups on measures including BDI, HAMD, HAMA, and the Generalized Anxiety Disorder-7 [74]. Detari et al. (2023) interviewed 14 healthcare professionals - coaches, physicians, researchers, and physical therapists - on their treatment approaches for managing NMS in musician’s dystonia. Strategies varied widely from addressing underlying perfectionism to motor retraining exercises [75]. O’Connor et al. (2023) surveyed 118 participants with CD, finding that illness perception and coping strategies accounted for 59% of the variance in anxiety, and 61% of the variance in depression and health-related QoL, highlighting the importance of these factors in the psychological adjustment to CD symptoms [76]. Zetterberg et al. (2024) interviewed patients with CD to explore their experiences with symptom management, identifying the use of coping strategies, acceptance of their condition, and adherence to BoNT treatments as key factors contributing to improved wellbeing. Common frustrations included the impact of stress on symptom severity, negative self-image leading to avoidant behaviors, and persistent pain and fatigue. These findings offer valuable insights into the patient experience and suggest areas of focus in multidisciplinary treatment strategies [77].
Discussion
This paper provides a comprehensive review of recent studies on NMS treatment in dystonia published within the past 5 years. The results regarding the impact of DBS on mood symptoms are inconsistent. Some studies (n = 105) report no significant improvement [46, 50, 51, 53, 56], while others (n = 109) indicate marked improvements [45, 52, 54, 57]. In direct comparisons of STN vs. GPi stimulation, STN-DBS was found to have a faster onset of mood symptom improvement in a mixed dystonia population, with superior effect in Meige syndrome compared to GPi-DBS [52, 57]. While DBS improved subjective measures of pain in patients with generalized dystonia, objective assessments of pain and sensory thresholds did not change post-DBS [54, 55, 57]. Additionally, cognitive function was generally unaffected by DBS across GPi, STN, and Vim targets [45, 50, 51, 57]. Although verbal fluency may improve with GPi-DBS, further research involving larger sample sizes is needed to substantiate this finding [56]. Finally, sleep quality does not appear to respond to DBS [50–52].
Ablative therapies, including radiofrequency or focused ultrasound of the PTT, show promise in targeting both motor and NMS, including mood and pain. However, the potential for significant adverse events, along with the unknown duration of therapeutic benefit, currently limits the recommended use of these procedures [47, 48].
BoNT consistently improves pain in CD and OMD. Mood consistently shows a transient response to BoNT during the injection cycle, but without long-term benefits. Sleep and cognitive outcomes do not show clear responses to BoNT, and additional evidence is needed to determine consistent effects. The limited improvement of NMS has been suggested as a major contributor to BoNT treatment dissatisfaction, as well as treatment failure and dropout rates [78].
Multifaceted treatment approaches - including light exercise, physical therapy and psychotherapy - under the guidance of healthcare professionals with expertise in dystonia, may enhance symptom management and improve QoL for patients [70, 73, 74, 77].
Theories of overlapping motor and nonmotor network dysfunction suggest that motor treatments could potentially influence NMS [6, 7]. While this hypothesis is plausible and offers practical benefits, evidence supporting a sustained and clinically significant NMS responses to motor treatments remains inconclusive. The findings of this systematic review do not clarify whether the NMS of dystonia are primary features of the disease or secondary to motor symptoms. It remains likely that both factors contribute to patients’ experience of dystonia-related NMS. Similar to Parkinson’s disease, a tailored approach addressing each patient’s specific combination of non-motor features, with symptom-specific interventions, may be necessary for optimal management.
Risk of bias
There were some notable risks of bias impacting this systematic review. The review protocol was not published, and was expanded after the initial PubMed review to include the other databases. Reporting bias remains a possibility in that non-significant results may have been overlooked in our selection and presentation of results. Within the PubMed search results, evidence selection bias is highly likely, in that studies with negative outcomes may not have been published. Within the primary studies, common biases included performance bias, given the lack of a control arm in most reports, and outcome reporting bias, especially as NMS were mostly secondary outcomes and non-significant findings were less likely to be included for publication. Figure 2 illustrates the presence of bias within the primary studies.
FIGURE 2
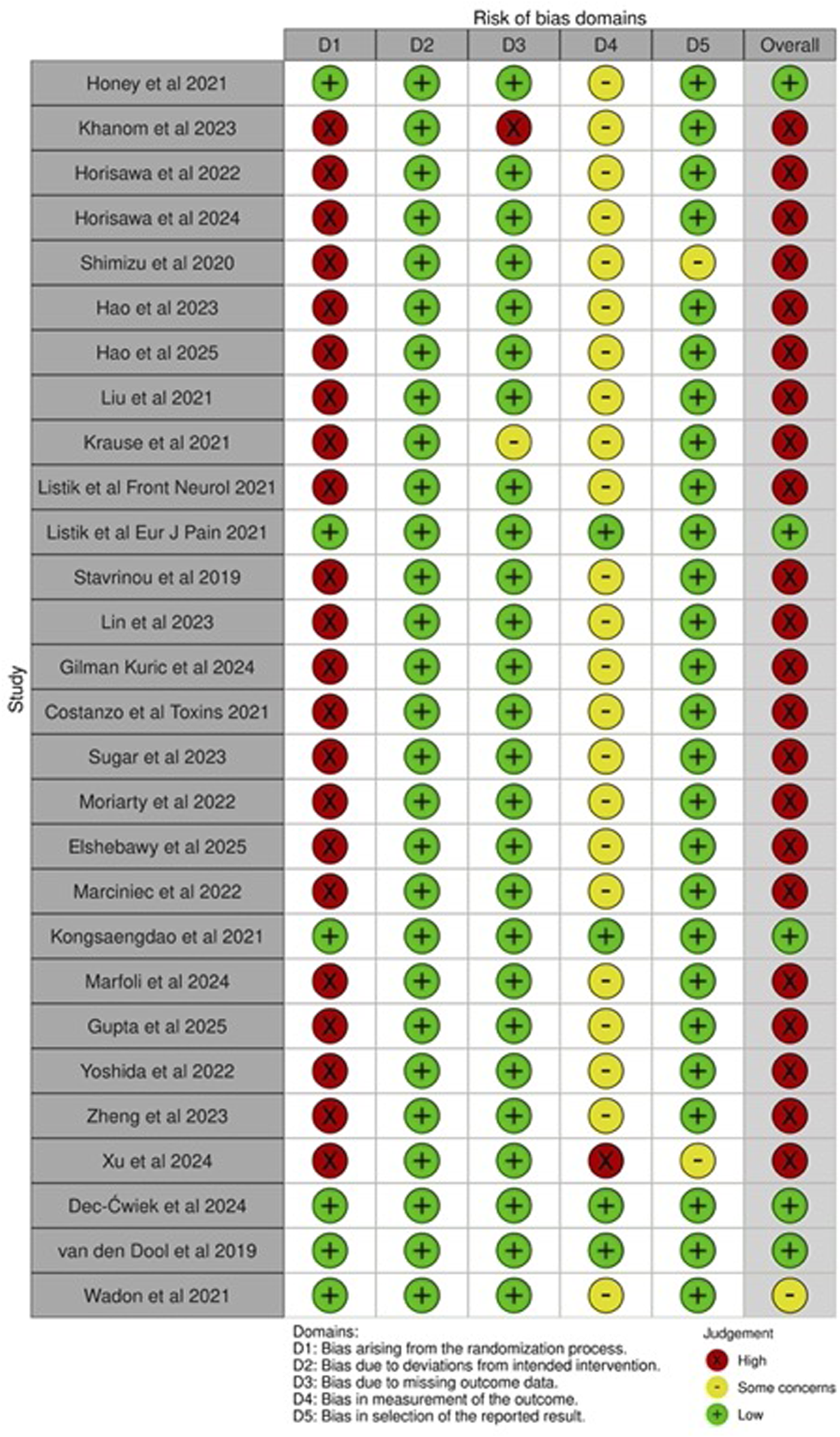
Risk of bias table [79]. See Supplementary Material S1 for raw table and justification. Presence of bias as related to non-motor symptom measurement and outcomes reporting, specifically assessed. The reviewed literature was found to have an overall high risk of bias due to the lack of control groups and randomization, and unblinded participants and outcomes assessors. See Supplementary Table S1 for justification.
Limitations and controversies
The overall quality of the studies reviewed was limited by small sample sizes and the absence of control groups. Additionally, the systematic analysis of the literature was limited by the use of only two keywords and future reviews should include specific NMS terms as keywords to capture a larger number of reports. Meta-analysis of reviewed data is precluded by the heterogeneous use of scales in NMS assessment, a well-documented challenge in dystonia research. The development and routine use of NMS-specific, disease-specific scales has been proposed to address this issue, similar to their application in Parkinson’s disease [8]. Several validated scales have been developed for distinct dystonia subgroups, enabling standardized outcome reporting. However, these scales generally focus on motor symptoms, limiting their capacity to comprehensively assess NMS outcomes. For example, the TWSTRS-Pain subscale used in CD populations may be less informative than other, more in-depth pain measures like the McGill Pain Questionnaire. Similarly, the TWSTRS-Psych may not capture the nuances of anxiety and depression as effectively as the Beck and Hamilton inventories, or the STAI. The growing recognition of NMS in dystonia is relatively recent, and the field of research remains in its early stages. However, progress has been made in the past 5 years, in this regard, including the development of a digital patient-centered outcome tool to measure NMS in CD, the Pain Scale for Adult-Onset Idiopathic Dystonia (PIDS) and its validation in CD, the Dystonia Pain Classification System (Dystonia-PCS), and the OMD Rating Scale (OMDRS), which includes NMS domains [80–83]. Future NMS scale development must strike a balance between specificity, depth, and ease of use in large study populations.
Most of the studies reviewed here primarily assessed motor symptoms, with NMS impact measured as secondary outcomes. This common theme underscores the need for high-quality research focusing on NMS improvement as a primary outcome in dystonia treatments. While still a subject of ongoing debate, there is a consensus that NMS in dystonia are distinct from motor symptoms and should be considered independent features of disease. Several studies support this view: da Silva-Júnior et al. (2022) found a high prevalence of NMS in patients with idiopathic isolated dystonia, without correlation to motor severity [3]. Li et al. (2020) reported that psychiatric symptoms in generalized dystonia did not correlate with motor severity, suggesting that NMS may be primary features of the disease [84]. Foley et al. (2017) concluded that cognitive symptoms and mood disorders in isolated dystonia occur independently of motor symptoms [85]. This view is further supported by studies identifying clinical subtypes of cervical dystonia based on NMS severity rather than motor symptom severity [86, 87].
Recent studies have identified distinct NMS-predominant subgroups within the cervical dystonia population. Wadon et al. (2022) and Costanzo et al. (2021) demonstrated two phenotypes: one motor-predominant, with fewer NMS, lower disability, and better QoL, and another NMS-predominant, with higher rates of depression, anxiety, sleep dysfunction, cognitive complaints, and pain [86, 87]. These subgroups did not differ in sex, motor severity, duration of dystonia, or the presence of tremor or alleviating maneuvers, though a potential age difference was noted, with the NMS-predominant group being older [86]. Further research is needed to verify these findings.
Regarding sex differences, while no significant differences between subgroups were observed in the aforementioned studies, other research has shown sex-based disparities in the prevalence of NMS in dystonia. Yang et al. (2021) found that women with blepharospasm had a higher frequency of NMS and were more likely to have multiple comorbid NMS [88]. Additionally, women with CD report worse health-related QoL compared to men [89]. The impact of sex on dystonia and NMS warrants further investigation, especially given the increased penetrance of CD in women, who are affected at least twice as often as men [90]. Notably, temporal discrimination threshold abnormalities, a hallmark feature of CD, are fully penetrant in unaffected first-degree female relatives, but only 40% penetrant in unaffected male relatives [89]. Further studies are needed to understand sex-based differences in disease susceptibility and phenotype.
In conclusion, NMS in dystonia significantly impact patient outcomes and require increased attention in both the clinic and research. An illustrative example of progress in this area is the work by Martino et al. (2023), who developed a care pathway for addressing mood symptoms in adult-onset isolated dystonia [91]. Further investigation is needed to refine dystonia subgroup phenotyping, explore sex differences, and standardize NMS rating scales across studies. These efforts would facilitate meta-analyses, improve the reproducibility of treatment outcomes, support the standardization of clinical practice, and improve patient outcomes.
Statements
Author contributions
DS and SP contributed to the conception and design of the study. DS contributed to the data collection, organization, and quality management. DS wrote the first draft of the manuscript. DS and SP edited and revised sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/dyst.2025.14545/full#supplementary-material
References
1.
Liang Y Lin J Hou Y Zhang L Ou R Li C et al Health-related quality of life in cervical dystonia using EQ-5D-5L: a large cross-sectional study in China. Front Neurol (2022) 13:895272. 10.3389/fneur.2022.895272
2.
Rafee S Al-Hinai M Douglas G Ndukwe I Hutchinson M . Mood symptoms in cervical dystonia: relationship with motor symptoms and quality of life. Clin Park Relat Disord (2023) 8:100186. 10.1016/j.prdoa.2023.100186
3.
Silva-Júnior Fp da Dos Santos Alves CO Silva SMCA Borges V Ferraz HB Rocha MSG et al High prevalence of self-reported non-motor symptoms and lack of correlation with motor severity in adult patients with idiopathic isolated dystonia. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol (2022) 43(2):1061–5. 10.1007/s10072-021-05452-3
4.
Tomic S Kuric TG Popovic Z Zubonja TM . Fatigue is related to depression in idiopathic dystonia. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol (2022) 43(1):373–8. 10.1007/s10072-021-05322-y
5.
Wadon ME Fenner E Kendall KM Bailey GA Sandor C Rees E et al Clinical and genotypic analysis in determining dystonia non-motor phenotypic heterogeneity: a UK Biobank study. J Neurol (2022) 269(12):6436–51. 10.1007/s00415-022-11307-4
6.
Rafee S O’Keeffe F O’Riordan S Reilly R Hutchinson M . Adult onset dystonia: a disorder of the collicular-pulvinar-amygdala network. Cortex J Devoted Study Nerv Syst Behav (2021) 143:282–9. 10.1016/j.cortex.2021.05.010
7.
Salazar Leon LE Sillitoe RV . Potential interactions between cerebellar dysfunction and sleep disturbances in dystonia. Dystonia Lausanne Switz (2022) 1:10691. 10.3389/dyst.2022.10691
8.
Stamelou M Edwards MJ Hallett M Bhatia KP . The non-motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain J Neurol (2012) 135(Pt 6):1668–81. 10.1093/brain/awr224
9.
Battistella G Simonyan K . Clinical implications of dystonia as a neural network disorder. Adv Neurobiol (2023) 31:223–40. 10.1007/978-3-031-26220-3_13
10.
Maione R Formica C Quartarone A Lo Buono V . The impact of non-motor symptoms on quality of life in cervical dystonia. J Clin Med (2023) 12(14):4663. 10.3390/jcm12144663
11.
Gowling H O’Keeffe F Eccles FJR . Stigma, coping strategies, distress and wellbeing in individuals with cervical dystonia: a cross-sectional study. Psychol Health Med (2024) 29(7):1313–30. 10.1080/13548506.2024.2305172
12.
Tarrano C Galléa C Delorme C McGovern EM Atkinson-Clement C Brochard V et al Psychiatric phenotype in neurodevelopmental myoclonus-dystonia is underpinned by abnormality of cerebellar modulation on the cerebral cortex. Sci Rep (2024) 14(1):22341. 10.1038/s41598-024-73386-9
13.
Menon V . Salience network. In: TogaAW, editor. Brain mapping. Academic Press (2015). p. 597–611. 10.1016/B978-0-12-397025-1.00052-X
14.
Goulden N Khusnulina A Davis NJ Bracewell RM Bokde AL McNulty JP et al The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage (2014) 99:180–90. 10.1016/j.neuroimage.2014.05.052
15.
Mahajan A Jankovic J Marsh L Patel A Jinnah HA Comella C et al Cervical dystonia and substance abuse. J Neurol (2018) 265(4):970–5. 10.1007/s00415-018-8840-9
16.
Ray S Kutty B Pal PK Yadav R . Sleep and other non-motor symptoms in patients with idiopathic oromandibular dystonia and meige syndrome: a questionnaire-based study. Ann Indian Acad Neurol (2021) 24(3):351–5. 10.4103/aian.AIAN_906_20
17.
Scorr LM Cho HJ Kilic-Berkmen G McKay JL Hallett M Klein C et al Clinical features and evolution of blepharospasm: a multicenter international cohort and systematic literature review. Dystonia Lausanne Switz (2022) 1:10359. 10.3389/dyst.2022.10359
18.
Defazio G Gigante AF Hallett M Berardelli A Perlmutter JS Berman BD et al Motor and psychiatric features in idiopathic blepharospasm: a data-driven cluster analysis. Parkinsonism Relat Disord (2022) 104:94–8. 10.1016/j.parkreldis.2022.10.008
19.
Ndukwe I O’Riordan S Walsh CB Hutchinson M . Trust the patient not the doctor: the determinants of quality of life in cervical dystonia. Front Neurol (2020) 11:991. 10.3389/fneur.2020.00991
20.
Zhang L Hou Y Lin J Yang J Cao B Wei Q et al Comprehensive analysis of non-motor symptoms and their association with quality of life in Writer’s cramp. Parkinsonism Relat Disord (2022) 100:37–40. 10.1016/j.parkreldis.2022.05.025
21.
Xavier Lde L Simonyan K . The extrinsic risk and its association with neural alterations in spasmodic dysphonia. Parkinsonism Relat Disord (2019) 65:117–23. 10.1016/j.parkreldis.2019.05.034
22.
Worthley A Simonyan K . Suicidal ideations and attempts in patients with isolated dystonia. Neurology (2021) 96(11):e1551–e1560. 10.1212/WNL.0000000000011596
23.
Bailey GA Matthews C Szewczyk-Krolikowski K Moore P Komarzynski S Davies EH et al Use of remote monitoring and integrated platform for the evaluation of sleep quality in adult-onset idiopathic cervical dystonia. J Neurol (2023) 270(3):1759–69. 10.1007/s00415-022-11490-4
24.
Han V Skorvanek M Smit M Turcanova Koprusakova M Hoekstra T van Dijk JP et al Prevalence of non-motor symptoms and their association with quality of life in cervical dystonia. Acta Neurol Scand (2020) 142(6):613–22. 10.1111/ane.13304
25.
Smit M Kamphuis ASJ Bartels AL Han V Stewart RE Zijdewind I et al Fatigue, sleep disturbances, and their influence on quality of life in cervical dystonia patients. Mov Disord Clin Pract (2017) 4(4):517–23. 10.1002/mdc3.12459
26.
Bailey GA Wadon ME Komarzynski S Matthews C Davies EH Peall KJ . Accelerometer-derived sleep measures in idiopathic dystonia: a UK Biobank cohort study. Brain Behav (2023) 13(9):e2933. 10.1002/brb3.2933
27.
Klingelhoefer L Kaiser M Sauerbier A Untucht R Wienecke M Mammadova K et al Emotional well-being and pain could be a greater determinant of quality of life compared to motor severity in cervical dystonia. J Neural Transm (2021) 128(3):305–14. 10.1007/s00702-020-02274-z
28.
Rosales RL Cuffe L Regnault B Trosch RM . Pain in cervical dystonia: mechanisms, assessment and treatment. Expert Rev Neurother (2021) 21(10):1125–34. 10.1080/14737175.2021.1984230
29.
Comella C Ferreira JJ Pain E Azoulai M Om S . Patient perspectives on the therapeutic profile of botulinum neurotoxin type A in cervical dystonia. J Neurol (2021) 268(3):903–12. 10.1007/s00415-020-10217-7
30.
Marciniec M Szczepańska-Szerej A Rejdak K . Cervical dystonia: factors deteriorating patient satisfaction of long-term treatment with botulinum toxin. Neurol Res (2020) 42(11):987–91. 10.1080/01616412.2020.1796430
31.
O’Connor S Hevey D Burke T Rafee S Pender N O’Keeffe F . A systematic review of cognition in cervical dystonia. Neuropsychol Rev (2024) 34(1):134–54. 10.1007/s11065-022-09558-z
32.
Monaghan R Cogley C Burke T McCormack D O'Riordan S Ndukwe I et al Non-motor features of cervical dystonia: cognition, social cognition, psychological distress and quality of life. Clin Park Relat Disord (2021) 4:100084. 10.1016/j.prdoa.2020.100084
33.
Mahady L White J Rafee S Yap SM O'Riordan S Hutchinson M et al Social cognition in cervical dystonia. Clin Park Relat Disord (2023) 9:100217. 10.1016/j.prdoa.2023.100217
34.
Burke T Monaghan R McCormack D Cogley C Pinto-Grau M O'Connor S et al Social cognition in cervical dystonia: a case-control study. Clin Park Relat Disord (2020) 3:100072. 10.1016/j.prdoa.2020.100072
35.
Rafee S Diepman M McCormack D Monaghan R Fearon C Hutchinson M et al A comprehensive cognitive analysis of cervical dystonia: a single centre study. Clin Park Relat Disord (2023) 9:100226. 10.1016/j.prdoa.2023.100226
36.
Tarrano C Galléa C Delorme C McGovern EM Atkinson-Clement C Barnham IJ et al Association of abnormal explicit sense of agency with cerebellar impairment in myoclonus-dystonia. Brain Commun (2024) 6(2):fcae105. 10.1093/braincomms/fcae105
37.
Delorme C Roze E Grabli D Mayer JM Degos B Vidailhet M et al Explicit agency in patients with cervical dystonia: altered recognition of temporal discrepancies between motor actions and their feedback. PloS One (2016) 11(8):e0162191. 10.1371/journal.pone.0162191
38.
Darby RR Joutsa J Burke MJ Fox MD . Lesion network localization of free will. Proc Natl Acad Sci U S A (2018) 115(42):10792–7. 10.1073/pnas.1814117115
39.
Sperduti M Delaveau P Fossati P Nadel J . Different brain structures related to self- and external-agency attribution: a brief review and meta-analysis. Brain Struct Funct (2011) 216(2):151–7. 10.1007/s00429-010-0298-1
40.
Coenen MA Spikman JM Smit M Klooster J Tijssen MAJ Gerritsen MJJ . Moving on with (social) cognition in idiopathic cervical dystonia. J Int Neuropsychol Soc JINS (2024) 30(5):464–70. 10.1017/S1355617723011426
41.
Eggink H Szlufik S Coenen MA Egmond MEvan Moro E Tijssen MAJ . Non-motor effects of deep brain stimulation in dystonia: a systematic review. Parkinsonism Relat Disord (2018) 55:26–44. 10.1016/j.parkreldis.2018.06.024
42.
Tyślerowicz M Kiedrzyńska W Adamkiewicz B Jost WH Sławek J . Cervical dystonia - improving the effectiveness of botulinum toxin therapy. Neurol Neurochir Pol (2020) 54(3):232–42. 10.5603/PJNNS.a2020.0021
43.
Erro R Picillo M Pellecchia MT Barone P . Improving the efficacy of botulinum toxin for cervical dystonia: a scoping review. Toxins (2023) 15(6):391. 10.3390/toxins15060391
44.
Peall KJ Berman BD Bruggemann N Defazio G Gimeno H Jinnah HA et al Non-motor symptoms in dystonia: from diagnosis to treatment. Dystonia (2023) 2:11860. 10.3389/dyst.2023.11860
45.
Honey CR Krüger MT Almeida T Rammage LA Tamber MS Morrison MD et al Thalamic deep brain stimulation for spasmodic dysphonia: a phase I prospective randomized double-blind crossover trial. Neurosurgery (2021) 89(1):45–52. 10.1093/neuros/nyab095
46.
Khanom AA Franceschini PR Lane S Osman-Farah J Macerollo A . Bilateral globus pallidus internus (GPi) deep brain stimulation for cervical dystonia: effects on motor and non-motor symptoms within 5 years follow. J Neurol Sci (2023) 452:120752. 10.1016/j.jns.2023.120752
47.
Horisawa S Kohara K Nonaka T Fukui A Mochizuki T Iijima M et al Unilateral pallidothalamic tractotomy at Forel’s field H1 for cervical dystonia. Ann Clin Transl Neurol (2022) 9(4):478–87. 10.1002/acn3.51532
48.
Horisawa S Saito R Qian B Hori H Kim K Murakami M et al Focused ultrasound pallidothalamic tractotomy in cervical dystonia: a pilot study. Mov Disord Off J Mov Disord Soc (2024) 40:132–40. 10.1002/mds.30030
49.
Shimizu T Maruo T Miura S Kimoto Y Ushio Y Goto S et al Burst spinal cord stimulation for the treatment of cervical dystonia with intractable pain: a pilot study. Brain Sci (2020) 10(11):827. 10.3390/brainsci10110827
50.
Hao Q Zheng W Zhang Z Liu YZ Ding H OuYang J et al Subthalamic nucleus deep brain stimulation in primary Meige syndrome: motor and non‐motor outcomes. Eur J Neurol (2023) 31(2):e16121. 10.1111/ene.16121
51.
Hao QP Zheng WT Zhang ZH Ding H Qin GB Liu YZ et al Deep brain stimulation and pallidotomy in primary Meige syndrome: a prospective cohort study. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol (2025) 46(1):207–17. 10.1007/s10072-024-07752-w
52.
Liu J Ding H Xu K Liu R Wang D Ouyang J et al Pallidal versus subthalamic deep-brain stimulation for meige syndrome: a retrospective study. Sci Rep (2021) 11(1):8742. 10.1038/s41598-021-88384-4
53.
Krause P Koch K Gruber D Kupsch A Gharabaghi A Schneider GH et al Long-term effects of pallidal and thalamic deep brain stimulation in myoclonus dystonia. Eur J Neurol (2021) 28(5):1566–73. 10.1111/ene.14737
54.
Listik C Cury RG Casagrande SCB Listik E Arnaut D Santiago N et al Improvement of non-motor symptoms and quality of life after deep brain stimulation for refractory dystonia: a 1-year follow-up. Front Neurol (2021) 12:717239. 10.3389/fneur.2021.717239
55.
Listik C Cury RG da Silva VA Casagrande SCB Listik E Link N et al Abnormal sensory thresholds of dystonic patients are not affected by deep brain stimulation. Eur J Pain Lond Engl (2021) 25(6):1355–66. 10.1002/ejp.1757
56.
Stavrinou LC Liouta E Boviatsis EJ Leonardos A Gatzonis S Stathis P et al Effect of constant-current pallidal deep brain stimulation for primary dystonia on cognition, mood and quality of life: results from a prospective pilot trial. Clin Neurol Neurosurg (2019) 185:105460. 10.1016/j.clineuro.2019.105460
57.
Lin S Shu Y Zhang C Wang L Huang P Pan Y et al Globus pallidus internus versus subthalamic nucleus deep brain stimulation for isolated dystonia: a 3-year follow-up. Eur J Neurol (2023) 30(9):2629–40. 10.1111/ene.15895
58.
Goetz CG Penn RD Tanner CM . Efficacy of cervical cord stimulation in dystonia. Adv Neurol (1988) 50:645–9.
59.
Gilman Kuric T Popovic Z Matosa S Sadikov A Groznik V Georgiev D et al Memory-guided saccades and non-motor symptoms improve after botulinum toxin therapy in cervical dystonia. J Clin Med (2024) 13(19):5708. 10.3390/jcm13195708
60.
Costanzo M Belvisi D Berardelli I Maraone A Baione V Ferrazzano G et al Effect of botulinum toxin on non-motor symptoms in cervical dystonia. Toxins (2021) 13(9):647. 10.3390/toxins13090647
61.
Sugar D Patel R Comella C González DA Gray G Stebbins GT et al The effect of botulinum toxin on anxiety in cervical dystonia: a prospective, observational study. Parkinsonism Relat Disord (2023) 114:105792. 10.1016/j.parkreldis.2023.105792
62.
Moriarty A Rafee S Ndukwe I O’Riordan S Hutchinson M . Longitudinal follow-up of mood in cervical dystonia and influence on age at onset. Mov Disord Clin Pract (2022) 9(5):614–8. 10.1002/mdc3.13457
63.
Elshebawy H Ramzy GM Salama M El-Jaafary S . Assessment of quality of life in patients with cervical dystonia and hemifacial spasm after botulinum toxin injections. Acta Neurol Belg (2025) 24:707–16. Published online February. 10.1007/s13760-025-02742-x
64.
Marciniec M Szczepańska-Szerej A Papuć E Rejdak K . Targeting pain in the long-term treatment of cervical dystonia with botulinum toxin A. Int J Neurosci (2022) 132(10):1026–30. 10.1080/00207454.2020.1860039
65.
Kongsaengdao S Arayawithchanont A Samintharapanya K Rojanapitayakorn P Maneeton B Maneeton N . Low-dose neubotulinum toxin A versus low-dose abobotulinum toxin A injection for the treatment of cervical dystonia: a multicenter, 48-week, prospective, double-blinded, randomized crossover design study. Toxins (2021) 13(10):694. 10.3390/toxins13100694
66.
Marfoli A Mameli F Aiello EN Ruggiero F Sandi AD Mellace D et al Does botulinum toxin affect psycho-social aspects in dystonia? J Neural Transm Vienna Austria (2024) 131(8):953–60. 10.1007/s00702-024-02785-z
67.
Gupta R Mehta S Balaini N Chakravarty K Singh J Mehta S et al Effect of Botulinum toxin on non-motor symptoms in adult-onset idiopathic focal/segmental dystonia. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol (2025) 46:2149–57. Published online January 30. 10.1007/s10072-025-08020-1
68.
Yoshida K . Effects of botulinum toxin therapy on health-related quality of life evaluated by the oromandibular dystonia rating scale. Toxins (2022) 14(10):656. 10.3390/toxins14100656
69.
Zheng H Wu L Tian S Liu M Zhan Q Yu X et al Effect of botulinum toxin type A on non-motor symptoms and quality of life in Meige syndrome. Front Neurol (2023) 14:1115482. 10.3389/fneur.2023.1115482
70.
van den Dool J Visser B Koelman JH Engelbert RH Tijssen MA . Long-term specialized physical therapy in cervical dystonia: outcomes of a randomized controlled trial. Arch Phys Med Rehabil (2019) 100(8):1417–25. 10.1016/j.apmr.2019.01.013
71.
Dec-Ćwiek M Sawczyńska K Porębska K Kubala M Witkowska M Żmijewska K . KinesioTaping: impact on non-motor symptoms in cervical dystonia patients treated with botulinum toxin injection. Neurol Neurochir Pol (2024) 58(1):127–33. 10.5603/PJNNS.a2023.0042
72.
Xu J Costanzo M Avanzino L Martino D Salehi P Standal S et al Vibro-tactile stimulation of the neck reduces pain in people with cervical dystonia: a proof-of-concept study. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol (2024) 45(10):4847–56. 10.1007/s10072-024-07561-1
73.
McCambridge A Meiring RM Bradnam LV . Physical activity, sedentary behavior, and barriers to exercise in people living with dystonia. Front Neurol (2019) 10:1121. 10.3389/fneur.2019.01121
74.
Wadon ME MacIver C Winter M Peall KJ . Internet-based cognitive behavioural therapy as a feasible treatment of adult-onset, focal, isolated, idiopathic cervical dystonia. Clin Park Relat Disord (2021) 5:100121. 10.1016/j.prdoa.2021.100121
75.
Détári A . Treating the musician rather than the symptom: the holistic tools employed by current practices to attend to the non-motor problems of musicians with task-specific focal dystonia. Front Psychol (2022) 13:1038775. 10.3389/fpsyg.2022.1038775
76.
O’Connor S Hevey D O’Keeffe F . Illness perceptions, coping, health-related quality of life and psychological outcomes in cervical dystonia. J Clin Psychol Med Settings (2023) 30(1):129–42. 10.1007/s10880-022-09851-2
77.
Zetterberg L Niemi Andersson E Åsenlöf P Nyholm D de Roos P Bring A . “I’m still the person I am. Not the body it has become.” an active but challenging life with cervical dystonia. Physiother Theor Pract (2022) 0(0):763–71. 10.1080/09593985.2024.2359495
78.
Sławek J Jost WH . Botulinum neurotoxin in cervical dystonia revisited - recent advances and unanswered questions. Neurol Neurochir Pol (2021) 55(2):125–32. 10.5603/PJNNS.a2021.0029
79.
McGuinness LA Higgins JPT . Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods (2021) 12(1):55–61. 10.1002/jrsm.1411
80.
Listik C Listik E de Paiva Santos Rolim F Meneses Cury Portela DM Perez Lloret S de Alves Araújo NR et al Development and validation of the dystonia-pain classification System: a multicenter study. Mov Disord Off J Mov Disord Soc (2023) 38(7):1163–74. 10.1002/mds.29423
81.
Bruno V Achen B Morgante F Erro R Fox SH Edwards MJ et al The pain in dystonia scale (PIDS)-Development and validation in cervical dystonia. Mov Disord Off J Mov Disord Soc (2023) 38(7):1175–86. 10.1002/mds.29452
82.
Yoshida K . Development and validation of a disease-specific oromandibular dystonia rating scale (OMDRS). Front Neurol (2020) 11:583177. 10.3389/fneur.2020.583177
83.
Pirio Richardson S Berman BD Hieshetter J Comella C Peterson DA Kilic-Berkmen G et al A digital patient-centered outcome tool for cervical dystonia. Dystonia (2024) 3:13478. 10.3389/dyst.2024.13478
84.
Li S Wang L Yang Y Qiao L Zhang D Wan X . Non-motor symptoms in Chinese patients with isolated generalized dystonia: a case-control study. Front Neurol (2020) 11:209. 10.3389/fneur.2020.00209
85.
Foley JA Vinke RS Limousin P Cipolotti L . Relationship of cognitive function to motor symptoms and mood disorders in patients with isolated dystonia. Cogn Behav Neurol Off J Soc Behav Cogn Neurol (2017) 30(1):16–22. 10.1097/WNN.0000000000000117
86.
Costanzo M Belvisi D Berardelli I Annalisa M Fabrizia D Viola B et al Motor and non-motor subtypes of cervical dystonia. Parkinsonism Relat Disord (2021) 88:108–13. 10.1016/j.parkreldis.2021.06.008
87.
Wadon ME Bailey GA Yilmaz Z Hubbard E AlSaeed M Robinson A et al Non-motor phenotypic subgroups in adult-onset idiopathic, isolated, focal cervical dystonia. Brain Behav (2021) 11(8):e2292. 10.1002/brb3.2292
88.
Yang J Zhang L Hou Y Wei Q Ou R Lin J et al Sex related differences in nonmotor symptoms of patients with idiopathic blepharospasm. Sci Rep (2021) 11(1):17856. 10.1038/s41598-021-97289-1
89.
Rafee S O’Riordan S Reilly R Hutchinson M . We must talk about sex and focal dystonia. Mov Disord Off J Mov Disord Soc (2021) 36(3):604–8. 10.1002/mds.28454
90.
Kilic-Berkmen G Scorr LM McKay L Thayani M Donsante Y Perlmutter JS et al Sex differences in dystonia. Mov Disord Clin Pract (2024) 11(8):973–82. 10.1002/mdc3.14059
91.
Martino D Nosratmirshekarlou E Cothros N Medina Escobar A Goodarzi Z . Development of a new care pathway for depression and anxiety in adult-onset isolated dystonia. Mov Disord Clin Pract (2023) 10(3):415–26. 10.1002/mdc3.13655
92.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. 10.1136/bmj.n71
Summary
Keywords
dystonia, outcomes, non-motor, symptoms, treatment
Citation
Sugar D and Pirio Richardson S (2025) Treating non-motor symptoms in dystonia: a systematic review. Dystonia 4:14545. doi: 10.3389/dyst.2025.14545
Received
26 February 2025
Accepted
18 June 2025
Published
30 June 2025
Volume
4 - 2025
Edited by
Giovanni Battistella, Massachusetts Eye and Ear Infirmary and Harvard Medical School, United States
Updates
Copyright
© 2025 Sugar and Pirio Richardson.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dana Sugar, dasugar@salud.unm.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.