Abstract
Cervical dystonia is the most common form of dystonia encountered in a movement disorders clinic. Botulinum toxin has been a long-established first line therapy. Several studies, including nearly two dozen randomized clinical trials, have shown that botulinum toxin is safe and effective in reducing the clinical severity of cervical dystonia. Longitudinal data have demonstrated decades of sustained benefit and safety. Although there is a potential for the development of botulinum toxin immunoresistance, this is quite rare, and partly determined by frequency of administration, cumulative dosage, and properties of the injected product. When immunoresistance does occur, switching to an alternative type of botulinum toxin (e.g., from type A to type B) usually restores the efficacy. In this evidence-based review we highlight the results of published double blind, placebo-controlled studies. We also briefly discuss injection techniques and some unmet needs, such as the development of practical assays to detect immunoresistance and longer-acting formulations of botulinum toxin.
Introduction
Botulinum toxin (BoNT) is a neurotoxin derived from the anaerobic bacterium, Clostridium botulinum (1–3). It is one of the most potent toxins found in nature, causing clinical botulism, a condition that leads to widespread paralysis and ultimately diaphragmatic respiratory failure (1, 4, 5). The toxin acts at the presynaptic nerve terminal by cleaving soluble N-ethylmaleimide-sensitive factor activating receptor (SNARE) proteins involved in docking of acetylcholine vesicles with the presynaptic membrane, thus preventing the release of acetylcholine leading to loss of muscle contraction, manifested by focal weakness when injected locally or generalized weakness, including respiratory, paralysis, when ingested in contaminated food (1, 6–8). There is also growing evidence that BoNT has a central effect, as well, by impacting antero- and retrograde transportation (9–12).
Capitalizing on the ability of BoNT to produce weakness by modulating the release of acetylcholine, this most potent biologic toxin known to man has emerged as one of the most multipurposed treatments for a large variety of neurologic and non-neurologic disorders (1, 7). In fact, Justinius Kerner, a German physician who initially described the effects of BoNT in 1817 suggested that it may have a therapeutic value in a variety of conditions such as St. Vitus dance, hypersalivation, and hyperhidrosis (1, 8). BoNT did not receive approval for human use by the United States Food and Drug Administration (FDA) until 1989, nearly a decade after the first publication of a double-blind, placebo-controlled trial establishing safety and efficacy of BoNT in cranio-cervical dystonia (13, 14). Since that time, BoNT has been approved for over a dozen indications including blepharospasm, hemifacial spasm, spasticity, detrusor overactivity, chronic migraines, hyperhidrosis, sialorrhea, and a variety of cosmetic indications (1, 15). In this review, we will focus on the use of BoNT in cervical dystonia (CD).
Methods of Literature Search
A comprehensive literature review of PubMed database was conducted with search criteria including the following key words: botulinum toxin treatment, cervical dystonia, randomized controlled trials (RCTs). Filters were set to RCTs between the years of 1980–2022. We excluded articles not published in the English language and those which were based on non-human subjects. The query resulted in 66 articles. Those were further reviewed to include both safety and efficacy data. Any ongoing trials or those that were beyond the scope of BoNT in treatment of CD were excluded.
Botulinum Toxin Products
Before reviewing the findings from the published studies of treatment with BoNT in CD, we wish to briefly describe the pharmacology and properties of the different formulations of BoNT as this is critical to the interpretation of the published results. Structurally, BoNT is composed 100 kD heavy chain and 50 kD light chain, linked by a disulfide bond (16). The heavy chain binds the complex to the presynaptic membrane and the light chain contains the active SNARE protein that cleaves either the intracellular synaptosomal-associated protein 25 (SNAP25) in the case of BoNT type A and E, or vesicle-associated membrane protein (VAMP), in the case of BoNT types B, D, and F (3). Some formulations of BoNT also contain non-toxic accessory proteins (NAPs) that include hemagglutinin and non-hemagglutinin, designed to prevent degradation (5, 17–19). The different forms of BoNT vary in the amount and types of NAPs and unique excipients (8, 16, 18).
Though most clinical trials have utilized BoNT type A and B, there are eight immunologically distinct forms of BoNT (A–H). There are four different preparations of BoNT that have been approved by the FDA: BoNTA onabotulinumtoxinA (Botox®), abobotulinumtoxinA (Dysport®), incobotulinumtoxinA (Xeomin®), and BoNTB rimabotulinumtoxinB (Myobloc®) (1, 6, 8). Other, novel, formulations are pending approval or in clinical trial, including a promising formulation of BoNTA called daxibotulinumtoxinA. In a phase 2 trial, which enrolled approximately 37 patients, daxibotulinumtoxinA was found to be safe and effective in the treatment of CD (20, 21). Results of the follow-up phase 3 clinical trial, with 301 patients, ASPEN-1, are pending full publication but have been presented at international meetings (22).
Generally, the effects of BoNT become evident within 1 week after injection, last 3–4 months, and then the benefits gradually wane. It has been proposed that the relatively short-lived effects are due to axonal sprouting at the presynaptic nerve terminal after injection with return of neuromuscular junction function that correlates with re-establishment of normal (baseline) muscle strength (1, 23, 24). With newer derivatives, such as daxibotulinumtoxin A, the effects have been found to last an average of 24 weeks, thus potentially reducing the number of injections needed per year to maintain optimal and sustained benefits. This may address a major concern for many patients—the wearing off of benefits before the next injection (25).
Immunogenicity
One of the potential risks associated with long-term BoNT use is the emergence of immunoresistance associated with the development of neutralizing antibodies (NABs) (1, 5, 21, 26). Several factors account for the immunogenicity of BoNT, including the unique properties of the formulation (some BoNT preparation have more accessory and stabilizing proteins that are inherently immunogenic), individual dose load and short inter-injection intervals (1, 5, 17, 26–29). The overall risk of immunogenicity varies among available products, ranging from 0%, as reported with incobotulinumtoxinA, to roughly 42% for rimabotulinumtoxinB (1, 5). BoNT resistance also varies by indication (5). The frequency of NABs is relatively low, but highest levels of NABs have been reported in patients treated for CD (30), followed by blepharospasm, hemifacial spasm and other conditions that generally require relatively low doses of BoNT, supporting the notion that immunogenicity is linked to total amount of toxin injected (5). AbobotulinumtoxinA (26) and especially, rimobotulinumtoxinB, having the highest risk of immunogenicity (18).
The possibility of immunoresistance should be considered when patients have at least two to three consecutive treatments without at least a 25% improvement. One of the biggest unmet needs in BoNT therapeutics is the lack of reliable assays to measure NABs. Currently available assays include the mouse protection assay (MPA) and mouse hemidiaphragm assay (MHDA) but these are difficult to perform and require sacrificing animals (1, 5). The unilateral brow injection is a good surrogate test for immunogenicity. It involves an injection of 20 U of BoNTA or 1,000 U of BoNTB in the medial eyebrow with assessment of ipsilateral procerus and corrugator muscle function at 1–2 weeks (1, 5). Other clinical tests for immunoresistance exist, but their discussion is beyond the scope of this article.
Cervical Dystonia
CD (also referred to as spasmodic torticollis) is a form of focal dystonia manifested by abnormal postures or movements produced by involuntary contractions of muscles in the neck, often associated with tremor (31–34) and pain (1, 6). In nearly a third of the cases of CD, regions outside of the neck are also involved with dystonia (35). Dystonia has an estimated prevalence of about 40 per 100,000 persons (36) and CD about 10 per 100,000, with an average age at onset of 40 years (1, 36–40). In isolated (primary) CD, about 12% of cases have a family history of dystonia, tremor or both (41, 42). There are many secondary causes of CD including neck injury (43). Although most patients have persistent, chronic symptoms, up to 20% experience spontaneous, but typically transient, remission, especially in younger individuals (1, 44). Patients with CD assume different combinations of head postures such as torticollis, laterocollis, retrocollis, and anterocollis, also categorized according to the “Col-Cap concept” (21,45). Patients with predominant anterocollis or retrocollis are often excluded from clinical trials of BoNT because this form of CD is more challenging to treat (21). In these patients using electromyography (EMG) or ultrasound to guide the injection may improve muscle targeting and outcomes (1).
An interesting phenomenon in CD, as in other forms of dystonia, is the alleviating maneuver, also referred to as “sensory trick” or “geste antogniste” (31, 46, 47). It is used in up to 90% of patients to reduce their dystonic posture and movement (46) which often complicates selection of muscles for injection with BoNT (46, 48). Alleviating maneuvers may not only “correct” abnormal postures but also can reduce dystonic head tremor (31, 49, 50). For example, arm raising has been found to effectively reduce head tremor in CD (31). Besides awareness of alleviating maneuvers, the clinician must be able to differentiate between contractions of the primary (agonist) versus compensatory (antagonistic) muscles in order to select the most appropriate muscles for targeting with BoNT (46, 47, 51).
The assessment of BoNT efficacy largely relies on clinical rating scales, such as the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) and Tsui score (52–54). Of the two, TWSTRS is the most frequently used and validated scale (1, 54). However, the TWSTRS scale is not without limitations, particularly when used in clinically mild CD (31, 46). Another limitation of TWSTRS is that it does not assess head tremor (55). Several studies have determined that the minimal clinically important change for improvement is 8–12-point reduction in total TWSTRS score (56).
Results
Several RCTs and long-term observational studies (57) have demonstrated the efficacy of BoNTA in the treatment of CD (1, 8). In this review we will focus on RCTs (Table 1) (58–82), although we acknowledge that placebo-controlled trials have important limitations, such as short duration of treatment, placebo effect, inflexible designs in which the therapy is not individualized, and other problems (83–85). Furthermore, open-label and observational longitudinal studies have provided important information besides efficacy, such as dosing and long-term safety (86–90).
TABLE 1
| Study | Study design and endpoint | Methods | Results | Class of evidencea |
|---|---|---|---|---|
| (58) | RDBPC assessing effectiveness of BoNTA in spasmodic torticollis | n = 21, patients received either BoNTA or placebo | BoNTA was found to be objectively and subjectively effective; 14 of 16 patients had significant pain reduction. No increased incidence of SEs in treatment group | II |
| (80) | RDBPC assessing BoNT in patients with torticollis | n = 20, patients stratified into 3 treatment and 1 placebo group and received 4 treatments each | 80% of patients reported subjective improvement with at least 1 dose, 55% reported substantial improvement. No objective improvement in torticollis; 4 patients reported transient dysphagia | II |
| (81) | Randomized, double-blind, placebo-controlled crossover study of BoNT in spasmodic torticollis | n = 20, 16 patients received BoNT and 4 received placebo | At 1 year follow up, statistically significant benefit in treatment arm (p = 0.04); dose related dysphagia | I |
| (59) | Randomized prospective, double-blind study assessing effectiveness of BoNTA to trihexyphenidyl in CD | n = 64, 32 patients received BoNTA with placebo tablet and 32 patients received trihexyphenidyl with placebo injection | BoNTA more effective than trihexyphenidyl in treatment of CD | I |
| (74) | RDBPC assessing the efficacy of BoNTB in BoNTA responsive and resistant patients with CD | n = 122, patients received either placebo, 2,500 U, 5,000 U or 10,000 U of BoNTB | Total TWSTRS scores were higher in all three dosage groups, dose dependent response observed. BoNTB found to be effective in treatment of CD | I |
| (73) | RDBPC assessing efficacy of BoNTB in patients with CD resistant to BoNTA | n = 77, 38 patients received placebo and 39 BoNTB | At weeks 4, 8, and 12 TWSTRS scores improved in the BoNTB treatment arm. BoNTB found to be effective in patients resistant to BoNTA | I |
| (104) | RDBPC for dose ranging in CD | n = 75, patients received either placebo, 250 U, 500 U or 1,000 U of ABOA | Good response was noted in 72% of 1,000 U arm, 44% of 500 U arm, 39% of 250 U arm and 10% of placebo; more side effects at 1,000 U dose compared to 250 U and placebo | I |
| (75) | RDBPC assessing efficacy of BoNTB in patients with CD previously treated with BoNTA | n = 109, patients received either placebo, 5,000 U or 10,000 U of BoNTB | TWSTRS scores significantly improved with 10,000 U treatment (p = 0.0016); BoNTB found to be safe at both doses | I |
| (70) | Summary of three clinical trials evaluating safety and efficacy of BoNTB in CD | Patients received 2,500–10,000 U of BoNTB | In all 3 trials, there was a statistically significant reduction in TWSTRS scores compared to placebo. In BoNTA responsive and resistant patients, BoNTB effects lasted 12–16 weeks. Side effects were mild, transient and anticipated. BoNTB is safe and effective in treatment of CD | I |
| (61) | RDBPC assessing the efficacy of ABOA 500 units in CD with Tsui score ≥ 9 | n = 68, patients randomized to either placebo or ABOA | There was a 49% reduction in pain in the treatment arm and 33% in placebo; 86% of treatment group and 42% of placebo were considered responders; adverse effects were reported in 42.9% of treatment and 27.3% of placebo groups; 500 U of ABOA is safe and effective for CD | I |
| (49) | Randomized, double-blind, crossover study comparing old ONA to new ONA in CD | n = 133, patients initially received 100–300 U, mean total dose of old ONA 155 U and new ONA 156 U | TWSTRS scores improved by −5.34 points in old ONA and −6.20 points in new ONA group. Nearly equivalent adverse events reported; new and old formulations of ONA have similar effects in treatment of CD | I |
| (63) | Randomized, double-blind trial assessing the efficacy of low dose BoNT in CD | n = 31, patients previously treated with at least 2 previous injections were treated with either 547 or 130 mouse units of ABOA | At 4 weeks, both groups had similar improvement in TWSTRS scores; marginally higher duration of effect in higher dosing group (65.8 days v. 57.4 days) | II |
| (64) | Randomized, double-blind trial comparing NT201 to ONA in CD | n = 463, 70–300 U of NT201 or ONA | TWSTRS score improved by −6.6 in NT201 and −6.4 in ONA groups from same mean starting score; 28.1% of NT201 and 24.1% of ONA groups reported SEs. Safety and tolerability were similar for both groups | I |
| (69) | Randomized, double-blind trial assessing BoNTA versus BoNTB in CD | n = 139, 74 received BoNTA at a max. dose of 250 U and 65 received BoNTB at a max. dose of 10,000 U | TWSTRS scores improved by −9.3 in the BoNTA and −10.2 in the BoNTB groups; duration of effect was longer in the BoNTA, on average 14 weeks and reduced incidence of adverse SEs | I |
| (65) | RDBPC assessing ABOA safety and efficacy in CD (in United States of America) | n = 80, patients either received 500 U ABOA or placebo | 38% of ABOA and 16% of placebo groups reported benefit; mean duration of ABOA was 18.5 weeks and increased reporting of SEs | I |
| (79) | Randomized, double-blind trial comparing BoNTA to BoNTB in CD | n = 111, 55 patients received BoNTA and 56 received BoNTB | TWSTRS score improved by −11 in BoNTA and −8.8 in BoNTB groups. Severe SEs similarly reported in both groups. Dry mouth more common in BoNTB group. Both formulations found to be effect in treating CD | I |
| (67) | Randomized prospective, double-blind trial comparing Prosigne to ONA | n = 24, patients received either 300 U of ONA or Prosigne; muscle selection was dependent on type of CD | ONA and Prosigne have the same safety profiles | II |
| (65) | RDBPC assessing the safety and efficacy of ABOA in CD | n = 111, 55 patients received 500 U ABOA and 61 received placebo | TWSTRS score improved −15.6 ± 2 in the treatment arm and −6.7 ± 2 in the placebo arm; ABOA was found to be safe and effective in CD | I |
| (68) | Prospective, RDBPC comparing INCA to placebo in CD | n = 233, patients were divided into placebo, 120 U INCA or 240 U INCA groups | TWSTRS score improved by −2.2, −9.9, −10.9, respectively. SEs including dysphagia, neck pain, and muscle weakness occurred at higher rates in the higher dose treatment group. INCA was found to be safe and effective | I |
| (78) | RDBPC, single dose study assessing RIMAB in CD (Japanese population) | Patients stratified into placebo, 2,500 U, 5,000 U or 10,000 U groups | At 4 weeks, TWSTRS scores improved in all treatment groups when compared to placebo; for disability and pain sub scores, only the 10,000 U showed significant improvement when compared to placebo. Dose dependent SEs reported | I |
| (77) | Randomized, double-blind crossover trial assessing ABOA to ONA at 2.5:1 ratio in CD | n = 103, patients randomly assigned to either treatment for 16 weeks, then after a 4-week washout period, were given the opposite treatment for another 16 weeks | ABOA at a ratio of 2.5:1 had similar efficacy and safety profile compared to ONA | I |
| (71) | RDBPC comparing ABOA to placebo in CD | n = 134, 89 patients received 500 U/2 dilution of ABOA and 45 received placebo | At 4-week endpoint, treatment group achieved statistically significant improvement in TWSTRS score (p = 0.001); most common SEs were dysphagia, muscle weakness, neck pain, headache. 500 U/2 dilution of ABOA is safe and effective in treating CD | I |
| (76) | RDBPC assessing the efficacy and safety of BoNTA in CD in dyskinetic cerebral palsy | n = 16, patients with dyskinetic cerebral palsy were injected with either BoNTA or saline (placebo) | At 4 weeks, TWSTRS score significantly improved in treatment arm (p = 0.028). Dysphagia occurred in 2 treatment patients and 1 placebo patient. BoNT was found to be safe and effective in treating pain and disability in CD | I |
| (47) | RDBPC phase 3b trial assessing efficacy of 2 ml ABOA injection at 12 weeks in CD | n = 134, 89 patients received ABOA (if treatment naïve received 250 U, otherwise 500 U) and 45 received placebo | At 12 weeks, mean TWSTRS score improved −7.1 in the treatment compared to −2 in the placebo group; Pain scale improved −1 vs. −0.2 in the treatment arm. Patients in treatment arm reported being “somewhat satisfied” more than placebo group. No new or serious SEs | I |
Randomized controlled trials of botulinum toxin in cervical dystonia.
Class of evidence was determined by the criteria outlined by the Quality and Standards subcommittee of the American Academy of Neurology (82).
RDBPC, randomized, double-blind, placebo-controlled study; CD, cervical dystonia; ST, spasmodic torticollis; BoNT, botulinum toxin (type A or B;, ABOA, abobotulinumtoxinA; INCA, incobotulinumtoxinA; ONA, onabotulinumtoxinA; RIMAB, rimabotulinumtoxinB; U = unit; SE, side effect; TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale.
While most studies involve BoNTA, trials assessing the safety and efficacy of BoNTB have found similar results (1, 70, 73, 75, 78, 90). There are few head-to-head trials comparing various formulations of BoNT. One study, involving 40 patients with CD, found no difference in efficacy between incobotulinumtoxinA and onabotulinumtoxinA at a 1:1 unit dose ratio (91). Another RCT, involving 103 patients, showed no significant difference between abobotulinumtoxinA and onabotulinumtoxinA at a dose ratio of 2.5:1 units (77). Two studies compared BoNTA with BoNTB at a dose ratios of 1 U BoNTA to 40 U of BoNTB (69) and at 1:66.7 (79), respectively, showed similar clinical efficacy and safety, however the frequency of dry mouth and injection pain was higher with BoNTB and the clinical effect has been found to last longer with BoNTA treatment in the study using a 1:40 ratio (69).
A Cochrane review conducted in 2016 that included three RCTs found no difference between the two types of BoNT but BoNTB had a higher frequency of dry mouth (92). Several other Cochrane reviews have demonstrated the efficacy of both, BoNTA and BoNTB, in the treatment of CD (93, 94), as well as the superiority of BoNTA to traditional oral therapies, such as trihexyphenidyl (95). Several investigators have reported higher risk of immunogenicity with BoNTB compared to BoNTA (96). Furthermore, treatment failure is more frequent with BoNTB compared to other formulations, likely due to increased immunogenicity, though the mechanism remains unclear (5, 18, 21).
Discussion
Numerous short-term RCT studies and long-term observational studies have demonstrated that the efficacy of BoNT can be sustained for decades along with continued safety (8, 15, 70, 73, 88, 97–107).
Dysphagia is the most frequently cited adverse effect of BoNT, reported in 5%–42% of patients, followed by muscle weakness in 3%–4%, injection pain 1%–9%, generalized weakness 0.3%, speech difficulties 0.3%, head drop 0.3%, rigidity 0.3%–3%, weight loss 0.3%, xerostomia (or dry mouth) 56%–71% (21). One side effect that it relatively common but rarely reported is flu-like symptoms occurring during the first few days after the injection in about 20% of patients (106). The mechanism of this transient adverse effect is not well understood but one study showed correlation with the interleukin inducible protein 10 (106).
Although BoNT has clearly improved the quality of life of patients with CD, up to 30% of patients discontinue treatment (108). The main reasons for discontinuation of therapy include change in provider, comorbid disease limiting treatment, advanced age, spontaneous improvement, adverse side effects and treatment failure (102, 108). The latter accounted for up to a 39% dropout rate from one study. Other studies have reported up to 26.1% drop out rate for primary, and 13.4% drop out rate for secondary non-responders (21). In other studies, common causes of discontinuation of therapy include a lack of response, short duration of effect, as well as inconvenience and cost of repetitive treatments, usually up to 4 times a year (109, 110). In a retrospective study of 568 patients treated with abobotulinumtoxinA for an average of 13 years, about 1.9% of patients were found to have partial secondary treatment failure per year, amounting to 14.5% of patients at 9 years (111). Factors contributing to secondary non-response include prior surgery to the area, prior side effects from BoNT, high mean doses of BoNT, and immunoresistance (5, 112).
One of the major limitations of BoNT is its relatively short duration (about 2–4 months) of benefit. In a survey of 209 patients, 88% reported re-emergence of symptoms in between treatments at an average of 10.5 weeks from injection (25). Generally, patients prefer treatments with longer injection intervals, which happens to be the best prognostic factor in overall satisfaction in symptoms control (25, 40). For these and other reasons, efforts at producing more effective and long-lasting formulations have been a priority in experimental therapeutics with BoNT in CD (20, 21).
Though most studies use primary endpoints of improvement in clinical rating scales, few evaluate for patient satisfaction or quality of life measures as surrogates for treatment efficacy. This has been highlighted by others, creating a need for improved rating systems that consider the complexity of achieving desirable results with minimal side effects (108).
Clinicians must always strive to optimize the response to BoNT by recognizing the full phenomenology of the patient’s CD and by making certain that they target the most relevant (agonist) muscles and avoid injecting compensatory (antagonist) muscles. Although EMG, ultrasound, kinematic guidance and other techniques have been found to improve accuracy of BoNT injections and possibly reduce the risk of BoNT-related side effects (45, 113–117), these techniques add cost, time, and discomfort and, therefore, should be used in selected cases rather than applied routinely to all BoNT injections (8, 38, 116).
Adjunctive treatment has an important role in overall patient satisfaction. Several studies have shown that anticholinergic drugs, baclofen, benzodiazepines and other muscles relaxants provide ancillary benefits in patients with CD treated with BoNT (42). Treating comorbid depression, anxiety, pain, fatigue and addressing stigmatization can all be therapeutic in the treatment of CD (108, 118). In one RCT studying the effects of physical therapy on CD, there was an improvement in TWSTRS score severity and pain by 31% in patients who received physical therapy in addition to BoNT injections versus 28% in those who only received BoNT (119). Though this study shows only marginal improvement, other studies have shown more robust benefits of physical therapy, particularly focusing on improving range of motion and preventing contractures (120). Another study found that the addition of physical therapy to BoNT injections extended the duration of improvement by 19.7 days with reduced pain and disability with activities of daily living (121).
Despite efforts to optimize efficacy by selecting appropriate targets, mitigating side effects, and reducing immunogenicity, some patients with CD remain refractory to BoNT. In these cases, advanced therapies such as deep brain stimulation (DBS) may provide benefit. A randomized, sham-controlled study conducted in Europe, which included 62 patients (32 assigned to neurostimulation vs. 30 assigned to sham stimulation), found that pallidal neurostimulation for 3 months is more effective than sham stimulation in reducing symptoms of CD (p = 0.0024) (122). A prospective pilot study found bilateral subthalamic nucleus stimulation improves dystonia, with a statically significant reduction in TWSTRS score, suggesting an alternative to pallidal DBS in the treatment of primary dystonia (123). In a smaller study of two patients who were previously implanted with pallidal targets, and subsequently received subthalamic nucleus DBS, one showed a 74% improvement and the other 84.3% improvement in TWSTRS scores (124). Finally, a metanalysis of RCTs evaluating DBS for dystonia found low-quality evidence to support the use of pallidal stimulation for the treatment of cervical, segmental or generalized moderate-to-severe cases of dystonia with improved functionality and reduced symptom severity (125).
Although BoNT is generally effective and safe for the treatment of CD, other adjunct therapies and physical therapy may be needed to optimize the response (Figure 1).
FIGURE 1
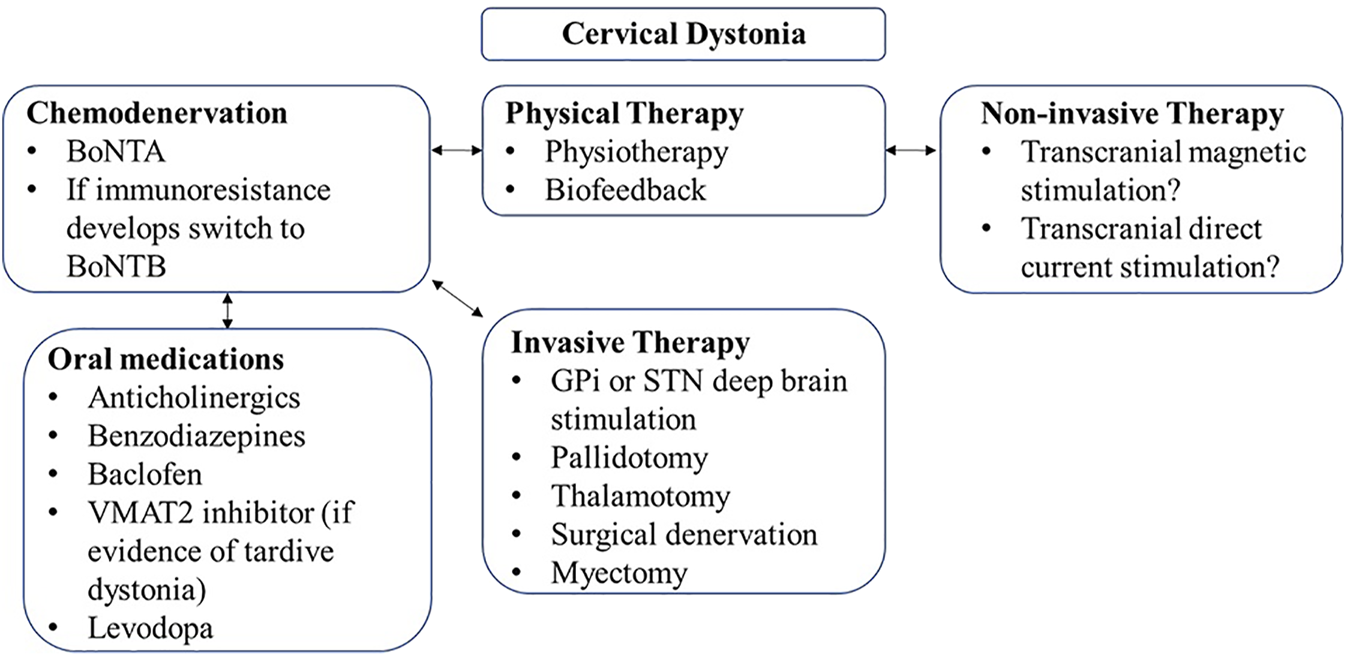
Treatment algorithm for cervical dystonia.
Conclusion
BoNT has been shown, through numerous open-label and controlled trials, to be safe and effective in the treatment of CD. These benefits, which clearly translate into improved quality of life, are usually sustained indefinitely. There are currently four formulations of BoNT (three types A and one type B) approved in the United States, but other formulations are available elsewhere and more are in development. The most important factor in favorable outcome following BoNT injection is the identification and appropriate selection of dose in the target muscle. It is likely that in the near future novel formulation of BoNT will be developed with improved properties such as longer duration of action, less diffusibility and immunogenicity, and lower cost which will lead to wider accessibility.
Statements
Author contributions
NH was responsible for data collection and manuscript writing. JJ was responsible for manuscript writing and editing.
Conflict of interest
JJ has received research or training grants from AbbVie Inc., CHDI Foundation, Dystonia Coalition, Emalex Biosciences, Inc., Medtronic Neuromodulation, Michael J Fox Foundation for Parkinson Research, National Institutes of Health, Parkinson’s Foundation, Revance Therapeutics, Inc., and Teva Pharmaceutical Industries Ltd. JJ has served as a consultant for AbbVie Inc., Aeon BioPharma, Neurocrine; Revance Therapeutics, and Teva Pharmaceutical Industries Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Anandan C Jankovic J . Botulinum Toxin in Movement Disorders: An Update. Toxins (2021) 13(1):42–73. 10.3390/toxins13010042
2.
Pirazzini M Rossetto O Eleopra R Montecucco C . Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol Rev (2017) 69(2):200–35. 10.1124/pr.116.012658
3.
Rossetto O Pirazzini M Fabris F Montecucco C . Botulinum Neurotoxins: Mechanism of Action. In: WhitcupSMHallettM, editors. Handbook of Experimental Pharmacology: Botulinum Toxin Therapy. Cham: Springer (2021). p. 35–48.
4.
Lonati D Schicchi A Crevani M Buscaglia E Scaravaggi G Maida F et al Foodborne Botulism: Clinical Diagnosis and Medical Treatment. Toxins (2020) 12(8):509. 10.3390/toxins12080509
5.
Bellows S Jankovic J . Immunogenicity Associated with Botulinum Toxin Treatment. Toxins (Basel) (2019) 11(9):491–513. 10.3390/toxins11090491
6.
Ferreira Camargo CH Ghizoni Teive HA . Use of Botulinum Toxin for Movement Disorders. Drugs Context (2019) 8:1–14. 10.7573/dic.212586
7.
Rossetto O Pirazzini M Montecucco C . Botulinum Neurotoxins: Genetic, Structural and Mechanistic Insights. Nat Rev Microbiol (2014) 12:535–49. Nature Publishing Group. 10.1038/nrmicro3295
8.
Jankovic J . Botulinum Toxin: State of the Art. Mov Disord (2017) 32:1131–8. John Wiley and Sons Inc. 10.1002/mds.27072
9.
Restani L Antonucci F Gianfranceschi L Rossi C Rossetto O Caleo M . Evidence for Anterograde Transport and Transcytosis of Botulinum Neurotoxin A (BoNT/A). J Neurosci (2011) 31(44):15650–9. 10.1523/JNEUROSCI.2618-11.2011
10.
Mazzocchio R Caleo M . More Than at the Neuromuscular Synapse: Actions of Botulinum Neurotoxin A in the central Nervous System. Neuroscientist (2015) 21(1):44–61. 10.1177/1073858414524633
11.
Weise D Weise CM Naumann M . Central Effects of Botulinum Neurotoxin—Evidence from Human Studies. Toxins (2019) 11:E21. 10.3390/toxins11010021
12.
Luvisetto S . Botulinum Neurotoxins in central Nervous System: An Overview from Animal Models to Human Therapy. Toxins (2021) 13:751. 10.3390/toxins13110751
13.
Jankovic J Orman J . Botulinum a Toxin for Cranial-Cervical Dystonia: A Double-Blind, Placebo-Controlled Study. Neurology (1987) 37:616–23. 10.1212/wnl.37.4.616
14.
Jankovic J . An Update on New and Unique Uses of Botulinum Toxin in Movement Disorders. Toxicon (2018) 147:84–8. 10.1016/j.toxicon.2017.09.003
15.
Hsiung GYR Das SK Ranawaya R Lafontaine AL Suchowersky O . Long-term Efficacy of Botulinum Toxin A in Treatment of Various Movement Disorders over a 10-year Period. Mov Disord (2002) 17:1288–93. 10.1002/mds.10252
16.
Kukreja Rv. Singh BR . Comparative Role of Neurotoxin-Associated Proteins in the Structural Stability and Endopeptidase Activity of Botulinum Neurotoxin Complex Types A and E. Biochemistry (2007) 46(49):14316–24. 10.1021/bi701564f
17.
Naumann M Boo LM Ackerman AH Gallagher CJ . Immunogenicity of Botulinum Toxins. J Neural Transm (2013) 120:275–90. 10.1007/s00702-012-0893-9
18.
Dressler D Bigalke H . Immunological Aspects of Botulinum Toxin Therapy. Expert Rev Neurother (2017) 17:487–94. Taylor and Francis Ltd. 10.1080/14737175.2017.1262258
19.
Kukreja R Chang TW Cai S Lindo P Riding S Zhou Y et al Immunological Characterization of the Subunits of Type A Botulinum Neurotoxin and Different Components of its Associated Proteins. Toxicon (2009) 53(6):616–24. 10.1016/j.toxicon.2009.01.017
20.
Jankovic J Truong D Patel AT Brashear A Evatt M Rubio RG et al Injectable DaxibotulinumtoxinA in Cervical Dystonia: A Phase 2 Dose-Escalation Multicenter Study. Mov Disord Clin Pract (2018) 5(3):273–82. 10.1002/mdc3.12613
21.
Marsili L Bologna M Jankovic J Colosimo C . Long-term Efficacy and Safety of Botulinum Toxin Treatment for Cervical Dystonia: a Critical Reappraisal. Expert Opin Drug Saf (2021) 20:695–705. Taylor and Francis Ltd. 10.1080/14740338.2021.1915282
22.
Jankovic J Comella C Hauser RA Todd GM Patel AT Rubio RG et al A Phase 3 Trial Evaluating the Efficacy, Duration of Effect, and Safety of DaxibotulinumtoxinA for Injection in the Treatment of Cervical Dystonia. Neurology (2022) 96.
23.
de Paiva A Meunier FA Molgo´ J Aoki KR Dolly JO . Functional Repair of Motor Endplates after Botulinum Neurotoxin Type A Poisoning: Biphasic Switch of Synaptic Activity between Nerve Sprouts and Their Parent Terminals. Proc Natl Acad Sci U S A (1999) 96:3200–5. 10.1073/pnas.96.6.3200
24.
Rogozhin AA Pang KK Bukharaeva E Young C Slater CR . Recovery of Mouse Neuromuscular Junctions from Single and Repeated Injections of Botulinum Neurotoxin A. J Physiol (2008) 586(13):3163–82. 10.1113/jphysiol.2008.153569
25.
Comella C Ferreira JJ Pain E Azoulai M Om S . Patient Perspectives on the Therapeutic Profile of Botulinum Neurotoxin Type A in Cervical Dystonia. J Neurol (2021) 268(3):903–12. 10.1007/s00415-020-10217-7
26.
Albrecht P Jansen A Lee JI Moll M Ringelstein M Rosenthal D et al High Prevalence of Neutralizing Antibodies after Long-Term Botulinum Neurotoxin Therapy. Neurology (2019) 92(1):E48–54. 10.1212/wnl.0000000000006688
27.
Zouhair Atassi M Jankovic J Dolimbek BZ . Neutralizing Antibodies in Dystonic Patients Who Still Respond Well to Botulinum Toxin Type A. Neurology (2008) 71:1040; author reply 1040-1. 10.1212/01.wnl.0000327865.05877.17
28.
Jankovic J Schwartz K . Response and Immunoresistance to Botulinum Toxin Injections. Neurology (1995) 45:1743–6. 10.1212/wnl.45.9.1743
29.
Brin MF Comella CL Jankovic J Lai F Naumann M Ahmed F et al Long-term Treatment with Botulinum Toxin Type A in Cervical Dystonia Has Low Immunogenicity by Mouse protection Assay. Mov Disord (2008) 23(10):1353–60. 10.1002/mds.22157
30.
Walter U Mühlenhoff C Benecke R Dressler D Mix E Alt J et al Frequency and Risk Factors of Antibody-Induced Secondary Failure of Botulinum Neurotoxin Therapy. Neurology (2020) 94(20):e2109–20. 10.1212/WNL.0000000000009444
31.
Cisneros E Vu JP Lee HY Chen Q Benadof CN Zhang Z et al Does Raising the Arms Modify Head Tremor Severity in Cervical Dystonia? Tremor Other Hyperkinet Mov (2021) 11(1):21. 10.5334/tohm.623
32.
Pal PK Samii A Schulzer SB Mak E Tsui JK . Head Tremor in Cervical Dystonia. Can J Neurol Sci (2000) 27(2):137–42. 10.1017/s0317167100052240
33.
Shaikh AG Beylergil SB Scorr L Kilic-Berkmen G Freeman A Klein C et al Dystonia and Tremor: A Cross-Sectional Study of the Dystonia Coalition Cohort. Neurology (2021) 96(4):e563–74. 10.1212/WNL.0000000000011049
34.
Fasano A Bove F Lang AE . The Treatment of Dystonic Tremor: A Systematic Review. J Neurol Neurosurg Psychiatry (2014) 85:759–69. BMJ Publishing Group. 10.1136/jnnp-2013-305532
35.
Kilic-Berkmen G Pirio Richardson S Perlmutter JS Hallett M Klein C Wagle-Shukla A et al Current Guidelines for Classifying and Diagnosing Cervical Dystonia: Empirical Evidence and Recommendations. Mov Disord Clin Pract (2022) 9(2):183–90. 10.1002/mdc3.13376
36.
Defazio G Berardelli A . Is Adult-Onset Dystonia a Rare Disease? Time for Population-Based Studies. Mov Disord (2021) 36:1119–24. John Wiley and Sons Inc. 10.1002/mds.28560
37.
Hong JS Sathe GG Niyonkuru C Munin MC . Elimination of Dysphagia Using Ultrasound Guidance for Botulinum Toxin Injections in Cervical Dystonia. Muscle Nerve (2012) 46(4):535–9. 10.1002/mus.23409
38.
Nijmeijer SWR Koelman JHTM Kamphuis DJ Tijssen MAJ . Muscle Selection for Treatment of Cervical Dystonia with Botulinum Toxin - A Systematic Review. Parkinsonism Relat Disord (2012) 18:731–6. 10.1016/j.parkreldis.2012.04.005
39.
Misra VP Colosimo C Charles D Chung TM Maisonobe P Om S et al INTEREST IN CD2, a Global Patient-Centred Study of Long-Term Cervical Dystonia Treatment with Botulinum Toxin. J Neurol (2018) 265(2):402–9. 10.1007/s00415-017-8698-2
40.
Colosimo C Charles D Misra VP Maisonobe P Om S Abdulnayef A et al How Satisfied Are Cervical Dystonia Patients after 3 Years of Botulinum Toxin Type A Treatment? Results from a Prospective, Long-Term Observational Study. J Neurol (2019) 266(12):3038–46. 10.1007/s00415-019-09527-2
41.
Chan J Brin MF Fahn S . Idiopathic Cervical Dystonia: Clinical Characteristics. Mov Disord (1991) 6(2):119–26. 10.1002/mds.870060206
42.
Balint B Mencacci NE Valente EM Pisani A Rothwell J Jankovic J et al Nat Rev Dis Primers (2018) 4(1):25. 10.1038/s41572-018-0023-6
43.
Jankovic J . Botulinum Toxin in Clinical Practice. J Neurol Neurosurg Psychiatry (2004) 75(7):951–7. 10.1136/jnnp.2003.034702
44.
Mainka T Erro R Rothwell J Kühn AA Bhatia KP Ganos C . Remission in Dystonia – Systematic Review of the Literature and Meta-Analysis. Parkinsonism Relat Disord (2019) 66:9–15. 10.1016/j.parkreldis.2019.02.020
45.
Jost WH Tatu L Pandey S Sławek J Drużdż A Biering-Sørensen B et al Frequency of Different Subtypes of Cervical Dystonia: a Prospective Multicenter Study According to Col–Cap Concept. J Neural Transm (2020) 127(1):45–50. 10.1007/s00702-019-02116-7
46.
Cisneros E Stebbins GT Chen Q Vu JP Benadof CN Zhang Z et al It’s Tricky: Rating Alleviating Maneuvers in Cervical Dystonia. J Neurol Sci (2020) 419:117205. 10.1016/j.jns.2020.117205
47.
Patel N Hanfelt J Marsh L Jankovic J , members of the Dystonia Coalition. Alleviating Manoeuvres (Sensory Tricks) in Cervical Dystonia. J Neurol Neurosurg Psychiatry (2014) 85(8):882–4. 10.1136/jnnp-2013-307316
48.
Comella CL Fox SH Bhatia KP Perlmutter JS Jinnah HA Zurowski M et al Development of the Comprehensive Cervical Dystonia Rating Scale: Methodology. Mov Disord Clin Pract (2015) 2(2):135–41. 10.1002/mdc3.12131
49.
Wissel J Müller J Ebersbach G Poewe W . Trick Maneuvers in Cervical Dystonia: Investigation of Movement-And Touch-Related Changes in Polymyographic Activity. Mov Disord (1999) 14(6):994–9. 10.1002/1531-8257(199911)14:6<994::aid-mds1013>3.0.co;2-k
50.
Frucht SJ . The Definition of Dystonia: Current Concepts and Controversies. Mov Disord (2013) 28(7):884–8. 10.1002/mds.25529
51.
Albanese A Asmus F Bhatia KP Elia AE Elibol B Filippini G et al EFNS Guidelines on Diagnosis and Treatment of Primary Dystonias. Eur J Neurol (2011) 18(1):5–18. 10.1111/j.1468-1331.2010.03042.x
52.
Ritter JM . Drug Regulation & Therapeutic Efficacy. Br J Clin Pharmacol (2008) 65:801–2. 10.1111/j.1365-2125.2008.03208.x
53.
Consky E Lang A . Clinical Assessment of Patients with Cervical Dystonia. In: JankovicJHallettM, editors. Therapy with Botulinum Toxin. New York: Marcel Dekker (1994). p. 211–37.
54.
Jost WH Hefter H Stenner A Reichel G . Rating Scales for Cervical Dystonia: A Critical Evaluation of Tools for Outcome Assessment of Botulinum Toxin Therapy. J Neural Transm (2013) 120:487–96. 10.1007/s00702-012-0887-7
55.
Comella CL Perlmutter JS Jinnah HA Waliczek TA Rosen AR Galpern WR et al Clinimetric Testing of the Comprehensive Cervical Dystonia Rating Scale. Mov Disord (2016) 31(4):563–9. 10.1002/mds.26534
56.
Dashtipour K Mari Z Jankovic J Adler CH Schwartz M Brin MF . Minimal Clinically Important Change in Patients with Cervical Dystonia: Results from the CD PROBE Study. J Neurol Sci (2019) 405:116413. 10.1016/j.jns.2019.07.031
57.
Jankovic J Adler CH Charles D Comella C Stacy M Schwartz M et al Primary Results from the Cervical Dystonia Patient Registry for Observation of OnabotulinumtoxinA Efficacy (CD PROBE). J Neurol Sci (2015) 349(1–2):84–93. 10.1016/j.jns.2014.12.030
58.
Tsui JK Eisen A Stoessl AJ Caln S Caln DB . Double-blind Study of Botulinum Toxin in Spasmodic Torticollis. Lancet (1986) 2:245–7. 10.1016/s0140-6736(86)92070-2
59.
Brans JW Lindeboom R Snoek JW Zwarts MJ van Weerden TW Brunt ERP et al Botulinum Toxin versus Trihexyphenidyl in Cervical Dystonia: a Prospective, Randomized, Double-Blind Controlled Trial. Neurology (1996) 46:1066–72. 10.1212/wnl.46.4.1066
60.
Poewe W Deuschl G Nebe A Feifel E Wissel J Benecke R et al What Is the Optimal Dose of Botulinum Toxin A in the Treatment of Cervical Dystonia? Results of a Double Blind, Placebo Controlled, Dose Ranging Study Using Dysport®. J Neurol Neurosurg Psychiatry. 1998;64(1):13–7. 10.1136/jnnp.64.1.13
61.
Wissel J Kanovsky P Ruzicka E Bares M Hortova H Streitova H et al Efficacy and Safety of a Standardised 500 Unit Dose of Dysport (clostridium Botulinum Toxin Type A Haemaglutinin Complex) in a Heterogeneous Cervical Dystonia Population: Results of a Prospective, Multicentre, Randomised, Double-Blind, Placebo-Controlled, Parallel Group Study. J Neurol (2001) 248:1073–8. 10.1007/s004150170028
62.
Naumann M Yakovleff A Durif F , BOTOX Cervical Dystonia Prospective Study Group. A Randomized, Double-Masked, Crossover Comparison of the Efficacy and Safety of Botulinum Toxin Type A Produced from the Original Bulk Toxin Source and Current Bulk Toxin Source for the Treatment of Cervical Dystonia. J Neurol (2002) 249:57–63. 10.1007/pl00007848
63.
Laubis-Herrmann U Fries K Topka H . Low-Dose Botulinum Toxin-A Treatment of Cervical Dystonia-A Double-Blind, Randomized Pilot Study. Eur Neurol (2002) 47:214–21. 10.1159/000057902
64.
Benecke R Jost WH Kanovsky P Ruzicka E Comes G Grafe S . A New Botulinum Toxin Type A Free of Complexing Proteins for Treatment of Cervical Dystonia. Neurology (2005) 64:1949–51. 10.1212/01.WNL.0000163767.99354.C3
65.
Truong D Brodsky M Lew M Brashear A Jankovic J Molho E et al Long-term Efficacy and Safety of Botulinum Toxin Type A (Dysport) in Cervical Dystonia. Parkinsonism Relat Disord (2010) 16(5):316–23. 10.1016/j.parkreldis.2010.03.002
66.
Truong D Duane DD Jankovic J Singer C Seeberger LC Comella CL et al Efficacy and Safety of Botulinum Type A Toxin (Dysport) in Cervical Dystonia: Results of the First US Randomized, Double-Blind, Placebo-Controlled Study. Mov Disord (2005) 20(7):783–91. 10.1002/mds.20403
67.
Quagliato EMAB Carelli EG Viana MA . A Prospective, Randomized, Double-Blind Study Comparing the Efficacy and Safety of Type A Botulinum Toxins Botox and Prosigne in the Treatment of Cervical Dystonia. Clin Neuropharmacol (2010) 33:22–6. 10.1097/WNF.0b013e3181c46f48
68.
Comella CL Jankovic J Truong DD Hanschmann A Grafe S , U.S. XEOMIN Cervical Dystonia Study Group. Efficacy and Safety of incobotulinumtoxinA (NT 201, XEOMIN ∗, Botulinum Neurotoxin Type A, without Accessory Proteins) in Patients with Cervical Dystonia. J Neurol Sci (2011) 308(1–2):103–9. 10.1016/j.jns.2011.05.041
69.
Comella CL Jankovic J Shannon KM Tsui J Swenson M Leurgans S et al Comparison of Botulinum Toxin Serotypes A and B for the Treatment of Cervical Dystonia. Neurology (2005) 65:1423–9. 10.1212/01.wnl.0000183055.81056.5c
70.
Lew M Brashear A Factor S . The Safety and Efficacy of Botulinum Toxin Type B in the Treatment of Patients with Cervical Dystonia: Summary of Three Controlled Clinical Trials. Neurology (2000) 55(12):29–35.
71.
Lew MF Brashear A Dashtipour K Isaacson S Hauser RA Maisonobe P et al A 500 U/2 mL Dilution of abobotulinumtoxinA vs. Placebo: Randomized Study in Cervical Dystonia. Int J Neurosci (2018) 128(7):619–26. 10.1080/00207454.2017.1406935
72.
Patel AT Lew MF Dashtipour K Isaacson S Hauser RA Ondo W et al Sustained Functional Benefits after a Single Set of Injections with abobotulinumtoxinA Using a 2-mL Injection Volume in Adults with Cervical Dystonia: 12-week Results from a Randomized, Double-Blind, Placebo-Controlled Phase 3b Study. PLoS ONE (2021) 16:e0245827. 10.1371/journal.pone.0245827
73.
Brin MF Lew MF Adler CH Comella C L Factor S A Jankovic J et al Safety and Efficacy of NeuroBloc (Botulinum Toxin Type B) in Type A-Resistant Cervical Dystonia. Neurology (1999) 53:1431–8. 10.1212/wnl.53.7.1431
74.
Lew MF Adornato BT Duane DD Dykstra DD Factor SA Massey JM et al Botulinum Toxin Type B: a Double-Blind, Placebo-Controlled, Safety and Efficacy Study in Cervical Dystonia. Neurology (1997) 49(3):701–7. 10.1212/wnl.49.3.701
75.
Brashear A Lew MF Dykstra DD Comella CL Factor S A Rodnitzky L et al Safety and Efficacy of NeuroBloc (Botulinum Toxin Type B) in Type A-Responsive Cervical Dystonia. Neurology (1999) 53:1439–46. 10.1212/wnl.53.7.1439
76.
Yi YG Kim K Yi Y Choi YA Leigh JH Bang MS . Botulinum Toxin Type a Injection for Cervical Dystonia in Adults with Dyskinetic Cerebral Palsy. Toxins (2018) 10(5):E203. 10.3390/toxins10050203
77.
Yun JY Kim JW Kim HT Chung SJ Kim JM Cho JW et al Dysport and Botox at a Ratio of 2.5:1 Units in Cervical Dystonia: A Double-Blind, Randomized Study. Mov Disord (2015) 30(2):206–13. 10.1002/mds.26085
78.
Kaji R Shimizu H Takase T Osawa M Yanagisawa N . [A Double-Blind Comparative Study to Evaluate the Efficacy and Safety of NerBloc® (rimabotulinumtoxinB) Administered in a Single Dose to Patients with Cervical Dystonia]. Brain Nerve (2013) 65(2):203–11.
79.
Pappert EJ Germanson T , Myobloc/Neurobloc European Cervical Dystonia Study Group. Botulinum Toxin Type B vs. Type A in Toxin-Naïve Patients with Cervical Dystonia: Randomized, Double-Blind, Noninferiority Trial. Mov Disord (2008) 23(4):510–7. 10.1002/mds.21724
80.
Gelb DJ Lowenstein DH Aminoff MJ . Controlled Trial of Botulinum Toxin Injections in the Treatment of Spasmodic Torticollis. Neurology (1989) 39(1):80–4. 10.1212/wnl.39.1.80
81.
Moore AP Blumhardt LD Moore LD Blumhardt AP . A Double-Blind Trial of Botulinum Toxin “A” in Torticollis, with One Year Follow up. J Neurol Neurosurg Psychiatry (1991) 54:813–6. 10.1136/jnnp.54.9.813
82.
Gross RA Johnson KC . Levels of Evidence Taking Neurology∗ to the Next Level. Neurology (2009) 72:8–10. 10.1212/01.wnl.0000342200.58823.6a
83.
Kostis JB Dobrzynski JM . Limitations of Randomized Clinical Trials. Am J Cardiol (2020) 129:109–15. 10.1016/j.amjcard.2020.05.011
84.
Möller HJ . Effectiveness Studies: Advantages and Disadvantages. Dialogues Clin Neurosci (2011) 13(2):199–207. 10.31887/dcns.2011.13.2/hmoeller
85.
Avins AL Cherkin DC Sherman KJ Goldberg H Pressman A . Should We Reconsider the Routine Use of Placebo Controls in Clinical Research?Trials (2012) 33:44. 10.1186/1745-6215-13-44
86.
Anderson TJ Rivest J Stell R Steiger J Cohen H Thompson PD et al Botulinum Toxin Treatment of Spasmodic Torticollis. J R Soc Med (1992) 85(9):524–9.
87.
Castelão M Marques RE Duarte GS Rodrigues FB Ferreira J Sampaio C et al Botulinum Toxin Type A Therapy for Cervical Dystonia. Cochrane Database Syst Rev (2017) 2017(12):CD003633. 10.1002/14651858.cd003633.pub3
88.
Kessler KR Skutta M Benecke R Kessler KR Skutta M Benecke R et al Long-term Treatment of Cervical Dystonia with Botulinum Toxin A: Efficacy, Safety, and Antibody Frequency. German Dystonia Study Group. J Neurol (1999) 246:265–74. 10.1007/s004150050345
89.
Mohammadi B Buhr N Bigalke H Krampfl K Dengler R Kollewe K . A Long-Term Follow-Up of Botulinum Toxin a in Cervical Dystonia. Neurol Res (2009) 31(5):463–6. 10.1179/174313209x405137
90.
Cullis PA O’Brien CF Truong DD Koller M Villegas TP Wallace JD . Botulinum Toxin Type B: an Open-Label, Dose-Escalation, Safety and Preliminary Efficacy Study in Cervical Dystonia Patients. Adv Neurol (1998) 78:227–30.
91.
Dressler D Tacik P Adib Saberi F . Botulinum Toxin Therapy of Cervical Dystonia: Comparing onabotulinumtoxinA (Botox∗) and incobotulinumtoxinA (Xeomin∗). J Neural Transm (2014) 121(1):29–31. 10.1007/s00702-013-1076-z
92.
Duarte GS Castelão M Rodrigues FB Marques RE Ferreira J Sampaio C et al Botulinum Toxin Type A versus Botulinum Toxin Type B for Cervical Dystonia. Cochrane Database Syst Rev (2016) 2016(10):CD004314. 10.1002/14651858.cd004314.pub3
93.
Marques RE Duarte GS Rodrigues FB Castelão M Ferreira J Sampaio C et al Botulinum Toxin Type B for Cervical Dystonia. Cochrane Database Syst Rev (2016) 2016(5):CD004315. 10.1002/14651858.cd004315.pub3
94.
Rodrigues FB Duarte GS Marques RE Castelão M Ferreira J Sampaio C et al Botulinum Toxin Type A Therapy for Cervical Dystonia. Cochrane Database Syst Rev (2020) 12(12):CD003633. 10.1002/14651858.cd003633.pub4
95.
Rodrigues FB Duarte GS Castelão M Marques RE Ferreira J Sampaio C et al Botulinum Toxin Type A versus Anticholinergics for Cervical Dystonia. Cochrane Database Syst Rev (2021) 2005(1):CD004312. 10.1002/14651858.cd004312.pub3
96.
Jankovic J Hunter C Dolimbek BZ Dolimbek GS Adler C H Brashear A et al Clinico-immunologic Aspects of Botulinum Toxin Type B Treatment of Cervical Dystonia. Neurology (2006) 67:2233–5. 10.1212/01.wnl.0000249308.66959.43
97.
Colosimo C Tiple D Berardelli A . Efficacy and Safety of Long-Term Botulinum Toxin Treatment in Craniocervical Dystonia: A Systematic Review. Neurotox Res (2012) 22(4):265–73. 10.1007/s12640-012-9314-y
98.
Ramirez-Castaneda J Jankovic J . Long-term Efficacy, Safety, and Side Effect Profile of Botulinum Toxin in Dystonia: A 20-year Follow-Up. Toxicon (2014) 90:344–8. 10.1016/j.toxicon.2014.07.009
99.
Mejia NI Dat Vuong K Jankovic J . Long-term Botulinum Toxin Efficacy, Safety, and Immunogenicity. Mov Disord (2005) 20(5):592–7. 10.1002/mds.20376
100.
Skogseid IM Kerty E . The Course of Cervical Dystonia and Patient Satisfaction with Long-Term Botulinum Toxin A Treatment. Eur J Neurol (2005) 12(3):163–70. 10.1111/j.1468-1331.2004.01053.x
101.
Fasano A Paramanandam V Jog M . Use of Abobotulinumtoxina in Adults with Cervical Dystonia: A Systematic Literature Review. Toxins (2020) 12(8):470. 10.3390/toxins12080470
102.
Jochim A Meindl T Mantel T Zwirner S Zech M Castrop F et al Treatment of Cervical Dystonia with Abo- and onabotulinumtoxinA: Long-Term Safety and Efficacy in Daily Clinical Practice. J Neurol (2019) 266(8):1879–86. 10.1007/s00415-019-09349-2
103.
Berman B Seeberger L Kumar R . Long-term Safety, Efficacy, Dosing, and Development of Resistance with Botulinum Toxin Type B in Cervical Dystonia. Mov Disord (2005) 20(2):233–7. 10.1002/mds.20290
104.
Poewe W Burbaud P Castelnovo G Jost WH Ceballos-Baumann AO Banach M et al Efficacy and Safety of abobotulinumtoxinA Liquid Formulation in Cervical Dystonia: A Randomized-Controlled Trial. Mov Disord (2016) 31(11):1649–57. 10.1002/mds.26760
105.
Vilanova TFDD Borges V Ferraz HB . Specific Characteristics of the Medical History of Swallowing before and after Application of Botulinum Toxin in Patients with Cervical Dystonia. Clinics (2019) 74:e776–15. 10.6061/clinics/2019/e776
106.
Baizabal-Carvallo JF Jankovic J Pappert E . Flu-like Symptoms Following Botulinum Toxin Therapy. Toxicon (2011) 58(1):1–7. 10.1016/j.toxicon.2011.04.019
107.
Bentivoglio AR di Stasio E Mulas D Cerbarano ML Ialongo T Laurienzo A et al Long-term Abobotulinumtoxin A Treatment of Cervical Dystonia. Neurotox Res (2017) 32:291–300. 10.1007/s12640-017-9737-6
108.
Tyślerowicz M Kiedrzyńska W Adamkiewicz B Jost WH Sławek J . Cervical Dystonia — Improving the Effectiveness of Botulinum Toxin Therapy. Neurol Neurochir Pol (2020) 54(3):232–42. 10.5603/PJNNS.a2020.0021
109.
Jinnah HA Comella CL Perlmutter J Lungu C Hallett M , Dystonia Coalition Investigators. Longitudinal Studies of Botulinum Toxin in Cervical Dystonia: Why Do Patients Discontinue Therapy?Toxicon (2018) 147:89–95. 10.1016/j.toxicon.2017.09.004
110.
Jinnah HA Goodmann E Rosen AR Evatt M Freeman A Factor S . Botulinum Toxin Treatment Failures in Cervical Dystonia: Causes, Management, and Outcomes. J Neurol (2016) 263(6):1188–94. 10.1007/s00415-016-8136-x
111.
Hefter H Spiess C Rosenthal D . Very Early Reduction in Efficacy of Botulinum Toxin Therapy for Cervical Dystonia in Patients with Subsequent Secondary Treatment Failure: A Retrospective Analysis. J Neural Transm (2014) 121(5):513–9. 10.1007/s00702-013-1127-5
112.
Ferreira JJ Colosimo C Bhidayasiri R Marti MJ Maisonobe P Om S . Factors Influencing Secondary Non-response to Botulinum Toxin Type A Injections in Cervical Dystonia. Parkinsonism Relat Disord (2015) 21:111–5. 10.1016/j.parkreldis.2014.09.034
113.
Kaymak B Kara M Gurcay E Ozcakar L . Sonographic Guide for Botulinum Toxin Injections of the Neck Muscles in Cervical Dystonia. Phys Med Rehabil Clin N Am (2018) 29(1):105–23. 10.1016/j.pmr.2017.08.009
114.
Ostergaard L Fuglsang-Frederiksen A Werdelin L Sjo O Winkel H . Quantitative EMG in Botulinum Toxin Treatment of Cervical Dystonia. A Double-Blind, Placebo-Controlled Study. Electroencephalogr Clin Neurophysiol (1994) 93(6):434–9. 10.1016/0168-5597(94)90150-3
115.
Brans JW Aramideh M Koelman JH Lindeboom R Speelman JD Ongerboer de Visser BW . Electromyography in Cervical Dystonia: Changes after Botulinum and Trihexyphenidyl. Neurology (1998) 51(3):815–9. 10.1212/wnl.51.3.815
116.
Kaymak B Gurcay E Ata AM Kara M Ozcakar L . Ultrasound Imaging and Guidance in the Management of Cervical Dystonia: A Caveat on the Compartmentalization of Sternocleidomastoid Muscle. Parkinsonism Relat Disord (2017) 43:127–8. 10.1016/j.parkreldis.2017.08.022
117.
Samotus O Lee J Jog M . Personalized Botulinum Toxin Type A Therapy for Cervical Dystonia Based on Kinematic Guidance. J Neurol (2018) 265(6):1269–78. 10.1007/s00415-018-8819-6
118.
Junker J Berman BD Hall J Wahba DW Brandt V Perlmutter JS et al Quality of Life in Isolated Dystonia: Non-motor Manifestations Matter. J Neurol Neurosurg Psychiatry (2021) 92(6):622–8. 10.1136/jnnp-2020-325193
119.
Hu W Rundle-Gonzalez V Kulkarni SJ Martinez-Ramirez D Almeida L Okun MS et al A Randomized Study of Botulinum Toxin versus Botulinum Toxin Plus Physical Therapy for Treatment of Cervical Dystonia. Parkinsonism Relat Disord (2019) 63:195–8. 10.1016/j.parkreldis.2019.02.035
120.
de Pauw J van der Velden K Meirte J van Daele U Truijen S Cras P et al The Effectiveness of Physiotherapy for Cervical Dystonia: A Systematic Literature Review. J Neurol (2014) 261(10):1857–65. 10.1007/s00415-013-7220-8
121.
Tassorelli C Mancini F Balloni L Pacchetti C Sandiri G Nappi G et al Botulinum Toxin and Neuromotor Rehabilitation: An Integrated Approach to Idiopathic Cervical Dystonia. Mov Disord (2006) 21(12):2240–3. 10.1002/mds.21145
122.
Volkmann J Mueller J Deuschl G Kühn AA Krauss JK Poewe W et al Pallidal Neurostimulation in Patients with Medication-Refractory Cervical Dystonia: A Randomised, Sham-Controlled Trial. Lancet Neurol (2014) 13(9):875–84. 10.1016/S1474-4422(14)70143-7
123.
Ostrem JL Racine CA Glass GA Grace JK Volz BMM Heath SL et al Subthalamic Nucleus Deep Brain Stimulation in Primary Cervical Dystonia. Neurology (2011) 76(10):870–8. 10.1212/WNL.0b013e31820f2e4f
124.
Gupta A . Subthalamic Stimulation for Cervical Dystonia. Acta Neurochir (Wien) (2020) 162(8):1879–81. 10.1007/s00701-020-04253-5
125.
Rodrigues FB Duarte GS Prescott D Ferreira J Costa J . Deep Brain Stimulation for Dystonia. In: Cochrane Database of Systematic Reviews (2019). p. 1. (CD012405).
Summary
Keywords
dystonia, treatment, cervical, torticollis, botulinum toxin
Citation
Hammoud N and Jankovic J (2022) Botulinum Toxin in the Treatment of Cervical Dystonia: Evidence-Based Review. Dystonia 1:10655. doi: 10.3389/dyst.2022.10655
Received
18 May 2022
Accepted
03 August 2022
Published
20 September 2022
Volume
1 - 2022
Edited by
Aasef Shaikh, Case Western Reserve University, United States
Reviewed by
Camilla Kilbane, Case Western Reserve University, United States
Shivam Om Mittal, Cleveland Clinic Abu Dhabi, United Arab Emirates
Updates
Copyright
© 2022 Hammoud and Jankovic.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph Jankovic, josephj@bcm.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.