Abstract
Background:
India witnessed three COVID-19 pandemic waves, each with various degrees of severity and clinical signs. The coronavirus strain and immunization status have a significant impact on the severity of COVID-19 infections. The current study intends to evaluate and compare the symptoms, severity, and breakthrough infections in vaccinated and unvaccinated individuals over the three waves of the pandemic.
Methods:
This was a retrospective survey study. A Google based questionnaire was used to collect data on demographics, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection status, re-infections, associated symptoms, severity, and vaccination status over three waves, post-COVID-19 sequelae, and willingness to receive a COVID-19 booster dose in the Indian population. The replies of 3,404 Indian participants were analysed using STATA 11.
Results:
Each wave showed a decrease in the number of symptomatic COVID-19 infections. However, fever and loss of smell/taste were identified as the most common symptoms in each wave. Clinical symptoms such as fever, weariness, and shortness of breath were shown to be considerably higher in vaccinated than unvaccinated individuals. The number of SARS-CoV-2 breakthrough infections increased between the second and third waves. Approximately 36.5% of people with protracted COVID-19 had previously received immunization after recovering from a natural COVID-19 illness. Overall, 34.8% of individuals were hesitant to take the COVID-19 booster dose.
Conclusion:
Increased symptoms in vaccinated individuals during the second wave, emphasizing the potential role of antibody-dependent augmentation. A considerable fraction (36.5%) of those with protracted COVID-19 infections had previously received vaccination after contracting the virus naturally. The fact that vaccine received after COVID-19 infection has been shown to be a risk factor for long-term COVID-19 emphasizes the need for vigilance in this specific subgroup.
Introduction
The emergence of a global pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), resulted in an unprecedented shift in human health, not only in terms of immediate disease but also prolonged symptoms in terms of long COVID-19 (Sharma et al., 2020). Additionally, in post-COVID-19 era, it continued to impact mental health, healthcare systems, economy, education, vaccine distribution and hesitancy, and social activities across the globe, indicating the essence of coordinated efforts from governments, businesses, communities, and individuals to build a resilient, equitable, and sustainable future (El-Shabasy et al., 2022). In India, the Wave-I began in March 2020 followed by the commencement of the Wave-II in mid-March 2021, attributed to the predominance of the delta variant, and the Wave-III at the beginning of mid-December 2021 with the preponderance of the omicron variant (Kapoor and Panda, 2022; Samarasekera, 2021; Kumar et al., 2022).
Furthermore, among the isolated seven different coronaviruses, SARS-CoV-2, SARS, and MERS caused severe respiratory syndromes, while other coronaviruses such as OC43, NL63, HKU1, and 229E were responsible for mild symptoms (Zhu et al., 2020; Zhang and Holmes, 2020). A total of five variants of concern (VOCs) of SARS-CoV-2 were reported during COVID-19, including Alpha, Beta, Gamma, Delta, and Omicron, along with different sub-lineages. Among the five VOCs, the Delta variant was reported as the most virulent and later it was replaced by the Omicron variant (B.1.1.529), which was reported to have 5-times more infectivity than the Delta variant (Cross, 2021). Omicron variant was classified into continuously evolving and emerging five lineages based on mutations in its genome: BA.1, BA.2, BA.3, BA.4, and BA.5 (Mohapatra et al., 2022; Zhou et al., 2023). Thus, three waves witnessed the preponderance of a single strain with many sub-strains, leading to an initial difference in symptoms and severity due to the infectivity bias of the strain, coupled with human diversity and natural immunity gained due to previous exposure. Recently, the World health organization (WHO) assigned two lineages (BA.2.86 and JN.1) of Omicron as variants of interest (VOI). The earliest sample for lineage BA.2.86 was documented from Israel and Denmark in July 2023 and was designated as VOI by WHO in November 2023. Likewise, the earliest sample documentation for lineage JN.1 was reported in August 2023 from Luxembourg, and WHO assigned it as VOI in February 2024 (World Health Organisation, 2023).
In India, individuals infected during the Wave-II had more prevalent clinical characteristics such as higher in older age groups, more ICU admissions, oxygen and ventilator requirements, and organ failure associated with increased mortality in comparison to Wave-I (Kapoor and Panda, 2022). In contrast, Wave-III showed less frequent patterns of illness and improved hospital outcomes, with a significantly lower rate of patient death (Kumar et al., 2022). The breakthrough COVID-19 infections, the infections occurring after the primary series of COVID-19 vaccination, were found to vary across different waves. Studies reported breakthrough SARS-CoV-2 infections, either with symptoms or without symptoms (Accorsi et al., 2022; Arora et al., 2022). However, there is a paucity of data on the comparative analysis of symptoms, severity, and breakthrough infections following either Covaxin or Covishield vaccination across the waves of COVID-19 in India. Further, the prevalence of asymptomatic or symptomatic COVID-19 infection depends on the individual’s immunity, COVID-19 strain, and the vaccination status of the infected individuals. However, according to the Centers for Disease Control and Prevention (CDC), the symptoms caused by the Omicron variant are like those of other variants (CDC, 2022). Remarkably, individuals who recovered from COVID-19 showed a significant functional impact of extended COVID-19 (Ziauddeen et al., 2022). Since the root causes of the prolonged COVID-19 symptoms were unknown, it was early to diagnose them as post-COVID-19 sickness (Perego et al., 2020).
The COVID-19 vaccination drive in India began from January 16, 2021, with two vaccines: Covishield (AZD-1222) manufactured by Serum Institute of India under license from Astra Zeneca, and Covaxin (BBV152), manufactured by Bharat Biotech, India, in association with the Indian Council of Medical Research (ICMR) (Ramasamy et al., 2021; Ella et al., 2021). Unexpectedly, the two doses of the COVID-19 vaccine could not provide sufficient prolonged immunity; therefore, many countries, including India, initiated the COVID-19 booster dose vaccination program initially for vulnerable people before being available for everyone.
In the present study, we investigated the clinical characteristics such as symptoms, severity, and breakthrough infections following either Covaxin or Covishield vaccination during three pandemic waves in India. The study also focused on the symptoms and characteristics of individuals experiencing post-COVID-19 sequelae. A further aim of this study was to shed light on the attitude and reasons of the individuals who were reluctant to use a booster dose of COVID-19. We believe that this study may be helpful for a better management of future outbreaks of COVID-19 or other similar infections.
Materials and methods
We conducted a pre-validated questionnaire-based survey study between March to June 2022. The survey included questions about the demographic status of patients infected with SARS-CoV-2, associated symptoms, and severity over three pandemic waves- Wave-I (March 2020 to December 2020), Wave-II (January 2021 to November 2021: predominance of delta variant) and Wave-III (after 01 December 2021: predominance of omicron variant), post-COVID-19 sequelae, vaccination status, re-infections, comorbidities, and willingness to take a COVID-19 booster dose.
Based on the questions relevant to symptoms, subjects were classified as asymptomatic and symptomatic. The symptomatic subjects were further classified on the basis of symptoms provided by them. The infection cases were classified as mild (with symptoms such as headache, body ache, loss of smell and taste, and myalgia, etc.); moderate (infection cases with fever as one of the symptoms along with other mild symptoms); and severe (which required oxygen support or hospitalization). The survey was conducted via two different means, viz. an online Google form and an offline survey conducted with the assistance of skilled healthcare professionals. The snowball sampling method was used to collect the responses. The data collected was a mixture of COVID-19-infected and non-infected populations. The detailed procedure for collecting offline forms has also been described earlier in Arora et al. (2022). The inclusion criteria comprise the subjects who are resident of India and above the age of 18 years. The subjects were also asked to give their consent before participating in the survey. The exclusion criteria involve the subjects who are non residents of India, below the age of 18 years and have taken vaccines other than Covaxin and Covishield. Also, the subjects who have not given the complete information were excluded from the present study.
For all the statistical analyses, the chi-square test, Fisher’s exact test, and Bonferroni correction were used for pairwise comparisons across the three waves (threshold of significant difference was 0.0167) and corresponding 98.33% confidence intervals were used. All data analyses were performed by implementing STATA 11 software and a P-value less than 0.05 was considered statistically significant for any difference.
Results
This Data from 3,404 participants was included, as shown in the flow chart consisting of the exclusion of missing data and the data fulfilling the inclusion criteria as depicted in Figure 1. Among the 1,297 participants, 2.4% (n = 31) developed COVID-19 twice either during Wave-I and later during Wave-II, or Wave-I and later during Wave-III, or Wave-II and later during Wave-III, as summarized in Supplementary Table S1. Furthermore, out of 1,297, 1,266 participants were divided based on the timeline of their infection into three waves as follows: Wave-I, 364 (28.75%); Wave-II, 663 (52.37%); and Wave-III, 239 (18.88%).
FIGURE 1
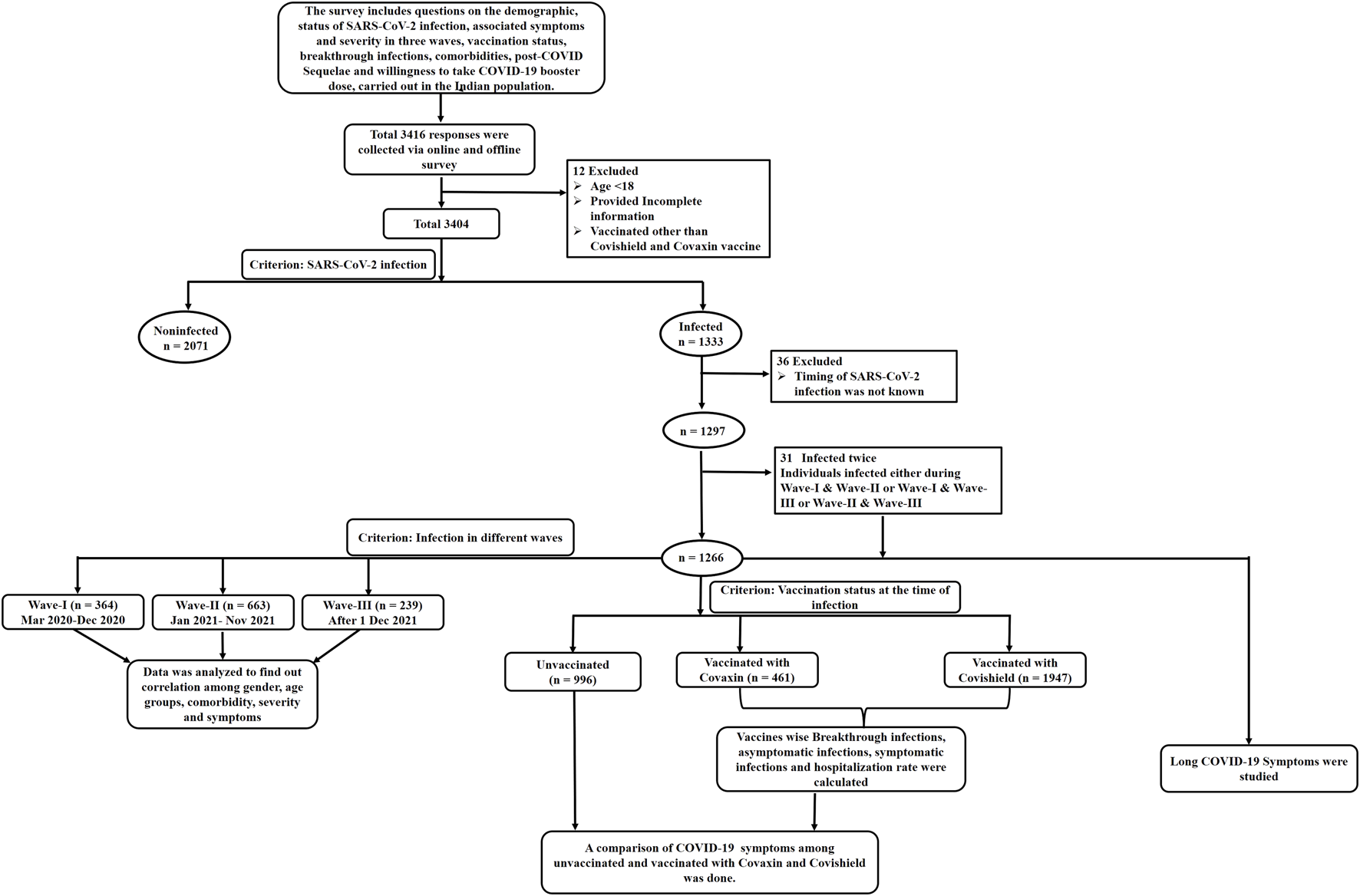
Flow Chart of the number of participants included in the study.
Demographic profile of participants
The participants in the study had a higher representation of respondents aged 18–40 years (73%), followed by those aged 41–60 years (21.9%), and the remaining 5.1% belonged to the age group 60 years and above (Supplementary Table S1). However, the genders were almost equally distributed among the participants. Most of the participants were from urban areas (69.4%), followed by suburban and rural areas (30.6%). About 33.5% of the participants were homemakers or retired people, followed by academicians (26.5%), workers (18.9%), frontline workers (8.9%), people working for corporate sectors (8.1%), and people working for government sectors (3.9%).
Among the total participants, 39.2% were affected by COVID-19, out of which the majority had experienced COVID-19 during the Wave-II (49.7%), as summarized in Supplementary Table S1. Among the COVID-19 affected participants, 86.3% experienced symptomatic infection during Wave-I, followed by Wave-II and Wave-III. A total of 71% of participants had received COVID-19 vaccine, with Covishield received by 80.9% and Covaxin by 19.1%. However, only 1.5% had received a COVID-19 booster dose.
Clinical characteristics of COVID-19 affected participants
The individuals who belonged to rural and sub-urban areas were infected highest (16.3%, 22.6%) during Wave-III as compared to Wave-II and Wave-I (Table 1). Notably, there was a higher percentage of COVID-19 individuals who belonged to urban areas during Wave-I and Wave-II (82.1% and 82.9%, respectively) in comparison to Wave-III (61.1%). On comparison of comorbidities across the waves, the percentage of participants who had common comorbidities such as diabetes, obesity, lung diseases, hypertension, and thyroid were higher during Wave-I (Table 1). We observed a significant difference in the proportion of symptomatic infection between Wave-I and Wave-III, and between Wave-II and Wave-III. Therefore, we further investigated the distribution of various symptoms. Fever was the most prevalent symptom during all three waves; moreover, the percentage of fever, shortness of breath, and headache was highest in Wave-I as compared to Wave-II and Wave-III. The proportion of symptoms such as abdominal pain, cold/running nose, and fatigue were highest in Wave-I as compared to Wave-II. In addition, the proportion of chest pain, headache, persistent cough, and loss of smell/taste symptoms was significantly reduced in Wave-III than in Wave-I or Wave-II. Shivering was found to be more prevalent in Wave-III than in Wave-I or Wave-II (Table 1). The difference across three waves for the symptoms such as skipped meals, diarrhoea, hoarse voice, anxiety, and nausea was found to be negligible. Since there were wide differences among the varied symptoms of COVID-19 during the three waves, we further explored the symptomatic and asymptomatic infections across the waves.
TABLE 1
| Variable | Wave-I (N = 364) | Wave-II (N = 663) | Wave-III (N = 239) | Wave-I vs Wave-II estimated difference [confidence interval] | P-Value | Wave-I vs Wave-III estimated difference [confidence interval] | P-Value | Wave-II vs Wave-III estimated difference [confidence interval] | P-Value |
|---|---|---|---|---|---|---|---|---|---|
| Age groups (Years) | |||||||||
| 18–40 | 249 (68.41) | 464 (70.09) | 151 (63.18) | −1.68 (−8.90 to 5.53) | 0.576 | 1.46 (−7.87–10.79) | 0.707 | 3.14 (−5.29–11.57) | 0.367 |
| 41–60 | 92 (25.27) | 167 (25.08) | 57 (23.85) | 0.19 (−6.59–6.97) | 0.947 | −0.67 (−9.37 to 8.03) | 0.854 | −0.86 (−8.75 to 7.03) | 0.793 |
| 61 and above | 23 (6.32) | 32 (4.83) | 31 (12.97) | 1.49 (−2.16–5.13) | 0.310 | −0.79 (−5.80 to 4.22) | 0.730 | −2.28 (−6.72 to 2.16) | 0.183 |
| Gender | |||||||||
| Female | 148 (40.66) | 318 (47.96) | 119 (49.79) | −7.30 (−15.01 to 0.42) | 0.025 | −9.13 (−19.02 to 0.76) | 0.027 | −1.83 (−10.86 to 7.20) | 0.627 |
| Male | 216 (59.34) | 345 (52.04) | 120 (50.21) | 7.30 (−4.15–15.01) | 0.025 | 9.13 (−0.76–19.02) | 0.027 | 1.83 (−7.20–10.86) | 0.627 |
| Places | |||||||||
| Rural | 13 (3.58) | 30 (4.55) | 39 (16.32) | −0.95 (−3.97 to 2.07) | 0.671 | −12.75 (−18.93 to 6.57) | <0.0001a | −11.8 (−17.84 to −5.76) | <0.0001a |
| Sub-urban | 52 (14.33) | 89 (13.48) | 54 (22.59) | 0.87 (−4.54–6.28) | 0.698 | −8.3 (−16.12 to −0.48) | 0.009a | −9.17 (−16.38 to −1.96) | 0.0009a |
| Urban | 298 (82.09) | 541 (81.97) | 146 (61.09) | 0.27 (−5.76–6.30) | 0.915 | 20.78 (11.82–29.74) | <0.0001a | 20.51 (12.15–28.87) | <0.0001a |
| Comorbidities present | 81 (22.25) | 142 (21.42) | 31 (12.97) | 0.83 (−4.46–6.12) | 0.758 | 9.28 (3.25–15.31) | 0.004 | 8.45 (3.17–13.73) | 0.004 |
| Diabetes | 21 (25.93) | 24 (16.90) | 5 (16.13) | 9.03 (3.70–14.36) | 0.001a | 9.80 (3.31–16.28) | 0.005 | 0.77 (−4.70 to 3.24) | 0.784 |
| Obesity | 20 (24.69) | 25 (17.61) | 3 (9.68) | 7.08 (1.79–12.37) | 0.007 | 15.01 (9.21–13.42) | <0.001a | 7.93 (3.19–12.67) | 0.004a |
| Lung diseases | 9 (11.11) | 11 (7.75) | 2 (6.45) | 3.36 (−0.46–7.18) | 0.071 | 4.66 (0.17–9.14) | 0.054 | 1.30 (−2.42 to 5.02 | 0.511 |
| Hypertension | 17 (20.99) | 18 (12.68) | 5 (16.13) | 8.31 (3.42–13.20) | 0.001a | 4.86 (−1.40–11.12) | 0.137 | −3.45 (8.76–1.86) | 0.182 |
| Thyroid | 13 (16.05) | 11 (7.75) | 3 (9.68) | 8.30 (4.01–12.59) | <0.001a | 6.37 (1.05–11.69) | 0.025 | −1.93 (−6.20 to 2.34) | 0.352 |
| Severity | |||||||||
| Hospitalization | 13 (3.57) | 30 (4.52) | 10 (4.18) | −1.01 (−4.24 to 2.22) | 0.467 | −0.48 (−4.57 to 3.61) | 0.777 | 0.53 (−3.31–4.37) | 0.747 |
| Symptoms present/symptomatic infection | 314 (86.26) | 556 (83.86) | 158 (66.11) | 2.98 (−2.52–8.48) | 0.206 | 20.43 (11.94–28.91) | <0.0001a | 20.45 (12.47–28.43) | <0.0001a |
| Fever | 182 (50.0) | 243 (36.65) | 80 (33.47) | 13.35 (5.64–21.06) | 0.0001a | 16.53 (6.90–26.15) | 0.0001a | 3.18 (−5.39–11.74) | 0.379 |
| Shortness of breath | 56 (15.38) | 73 (11.01) | 18 (7.53) | 4.37 (−1.01–9.75) | 0.043 | 7.85 (1.75 (13.94) | 0.004a | 3.48 (−1.53–8.50) | 0.126 |
| Chest pain | 34 (9.34) | 40 (6.03) | 4 (1.67) | 3.31 (−0.96–8.24) | 0.050 | 7.67 (3.51–11.82) | 0.0001a | 4.36 (1.39–7.33) | 0.007a |
| Headache | 113 (31.04) | 155 (23.38) | 38 (15.9) | 7.66 (0.65–14.67) | 0.008 | 15.14 (7.03–23.24) | <0.0001a | 8.29 (1.49–15.09) | 0.007a |
| Body ache | 102 (28.02) | 148 (22.32) | 41 (17.15) | 5.70 (−1.11–12.53) | 0.042 | 10.87 (27.59–18.98) | 0.002a | 5.17 (−1.83–12.17) | 0.092 |
| Skipped meals | 9 (2.47) | 9 (1.36) | 5 (2.09) | 1.11 (−1.11–3.33) | 0.195 | 0.38 (−2.57–3.32) | 0.761 | −0.73 (−3.19 to 1.73) | 0.434 |
| Abdominal pain | 11 (3.02) | 6 (0.9) | 6 (2.51) | 2.12 (−0.20–4.44) | 0.011a | 0.51 (−2.72–3.75) | 0.711 | −1.61 (-4.19 to 0.97) | 0.062 |
| Fatigue | 76 (20.88) | 92 (13.88) | 41 (17.15) | 7.00 (0.97–13.03) | 0.004a | 3.73 (−4.01–11.48) | 0.257 | −3.27 (−9.93 to 3.39) | 0.222 |
| Diarrhea | 17 (4.67) | 29 (4.37) | 9 (3.77) | 0.30 (−2.96–3.56) | 0.824 | 0.90 (−3.06–4.86) | 0.594 | 0.60 (−2.91–4.11) | 0.692 |
| Hoarse voice | 8 (2.2) | 12 (1.81) | 12 (5.02) | 0.39 (−1.83–2.61) | 0.665 | −2.82 (−6.67–10.29) | 0.059 | −3.21 (−6.81 to 0.39) | 0.008 |
| Cold/Running nose | 36 (9.89) | 33 (4.98) | 36 (15.06) | 4.91 (0.65–9.17) | 0.003a | −5.17 (−11.85 to 1.51) | 0.055 | −10.08 (−15.97 to −4.19) | <0.0001a |
| Anxiety | 24 (6.59) | 39 (5.88) | 10 (4.18) | 0.71 (−3.09–4.51) | 0.650 | 2.41 (−1.98–6.80) | 0.209 | 1.70 (−2.09–5.49) | 0.320 |
| Persistent cough | 93 (25.55) | 165 (24.89) | 22 (9.21) | 0.66 (−6.13–7.44) | 0.816 | 16.34 (9.27–23.40) | <0.0001a | 15.68 (9.66–21.70) | <0.0001a |
| Loss of smell/taste | 123 (33.79) | 201 (30.32) | 47 (19.67) | 3.57 (−3.74–10.87) | 0.239 | 14.12 (5.57–22.67) | 0.0002a | 10.65 (3.16–18.14) | 0.002a |
| Shivering | 7 (1.92) | 13 (1.96) | 25 (10.46) | −0.04 (−2.19 to 2.11) | 0.965 | −8.54 (−13.58 to −3.50) | <0.0001a | −8.50 (−13.41 to −3.59) | <0.0001a |
| Nausea | 10 (2.75) | 16 (2.41) | 9 (3.77) | 0.34 (−2.16–2.84) | 0.550 | −1.02 (−4.61 to 2.57) | 0.483 | −1.36 (−4.63 to 1.91) | 0.272 |
Clinical Characteristics of COVID-19 affected participants of the three waves.
98.33% confidence intervals were calculated after correction to significance level using Bonferroni method.
Proportion of symptomatic and asymptomatic SARS-CoV-2 infection
Of the 1,266 individuals with COVID-19, the proportion of asymptomatic infection increased, and the proportion of symptomatic infection declined from the Wave-I to the Wave-III, as depicted in Figure 2A. Since the asymptomatic infection were observed to be increased, approximately two-fold in the Wave-III (33.9%; 81/239), we assessed the vaccination status in individuals with asymptomatic (n = 238) and symptomatic infections (n = 1,028). A total of 30.7% of participants with asymptomatic infections had received 1st dose and 69.3% were unvaccinated. Likewise, 27.1% of participants with symptomatic infections had received 1st dose and 72.9% were unvaccinated (P > 0.05).
FIGURE 2
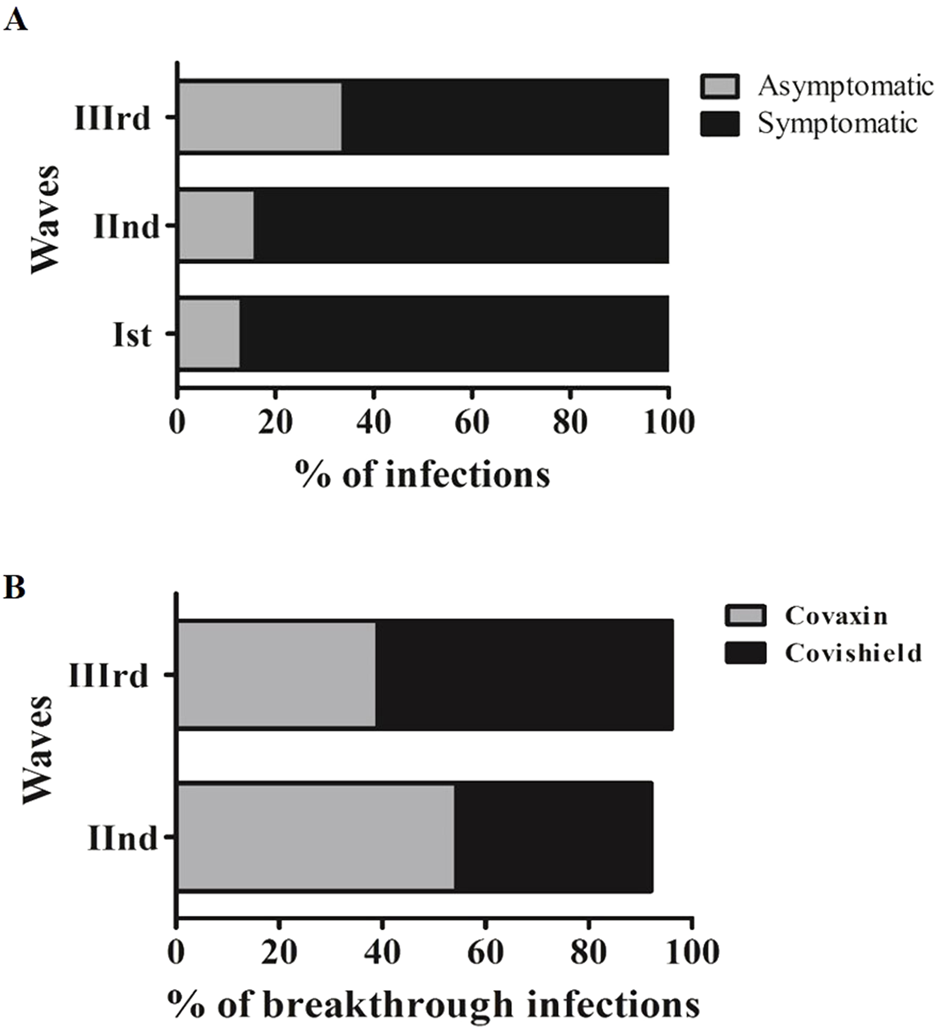
(A) Distribution of symptomatic, asymptomatic and vaccination status of asymptomatic participants among three waves. (B) A depiction of percentage breakthrough infection and clinical symptoms among participants during three waves.
Further, we investigated the rate of asymptomatic infections among the individuals who had a prior history of COVID-19 (n = 18). As the highest asymptomatic infections were experienced by COVID-19-infected individuals during Wave-III, we further bifurcated the information from these participants (Supplementary Table S2). Around 72.2% (n = 13; 13/18) individuals were found to be fully vaccinated with the COVID-19 vaccine at the time of Wave-III infection, while 27.8% (n = 5; 5/18) were unvaccinated. Among vaccinated individuals, 30.8% (n = 4; 4/13) had experienced asymptomatic infection during Wave-III and had a history of prior COVID-19 during Wave-I (n = 2) or Wave-II (n = 2 out of a total of 11 infected individuals during Wave-II). However, among unvaccinated individuals, 100% (n = 5; 5/5) had experienced symptomatic COVID-19 infections. Of note, those individuals who had a prior history of infections (n = 18), had experienced symptomatic COVID-19 infection and were unvaccinated (94.4%; 17/18) during their previous COVID-19 infection (Supplementary Table S2).
Clinical spectrum and severity of among symptomatic vaccinated and unvaccinated individuals
The characteristics and clinical spectrum of participants who had a symptomatic COVID-19 infection were divided based on their vaccination status as summarized in Table 2. Among the participants who had symptomatic COVID-19 infection, 27.1% (279/1,028) were vaccinated and 72.9% (749/1,028) were unvaccinated. Overall, among the participants with the symptomatic COVID-19 infection, the proportion of unvaccinated young age group participants was observed to be significantly higher than the other age groups (Table 2). On comparison of clinical symptoms among vaccinated and unvaccinated participants, fever, fatigue, shortness of breath, persistent cough, hoarse voice, and shivering were found to be significantly higher among the vaccinated participants. However, the difference between the other symptoms such as loss of smell/taste, headache, body ache, cold/running nose, chest pain, anxiety, and diarrhoea among vaccinated and unvaccinated participants was found to be negligible.
TABLE 2
| Parameters | Vaccinated (N = 279) | Unvaccinated (N = 749) | P-value |
|---|---|---|---|
| Gender | |||
| Males | 150 (53.76) | 409 (54.61) | 0.058 |
| Females | 129 (46.24) | 340 (45.39) | |
| Median Age (range) | |||
| 18–40 | 159 (56.99) | 546 (72.90) | <0.0001a |
| 41–60 | 84 (30.11) | 172 (22.96) | |
| 61 and above | 36 (12.90) | 31 (4.14) | |
| Comorbidities present | 58 (20.79) | 166 (22.16) | 0.225 |
| No. of Severe infection/hospitalization | 18 (6.45) | 34 (4.54) | 1.548 |
| Common Symptoms | |||
| Fever | 162 (58.06) | 341 (45.53) | 0.003a |
| Loss of taste and smell | 97 (34.77) | 274 (36.58) | 0.590 |
| Fatigue | 70 (25.09) | 138 (18.42) | 0.018a |
| Headache | 76 (27.24) | 227 (30.31) | 0.338 |
| Body ache | 79 (28.32) | 213 (28.44) | 0.969 |
| Shortness of breath | 50 (17.92) | 99 (13.22) | 0.0001a |
| Cold/Running nose | 32 (11.47) | 72 (9.61) | 0.380 |
| Persistent Cough | 27 (9.68) | 37 (4.94) | 0.001a |
| Chest pain | 17 (6.09) | 61 (8.14) | 0.502 |
| Hoarse voice | 13 (4.66) | 17 (2.27) | 0.043a |
| Anxiety | 20 (7.17) | 53 (7.08) | 0.959 |
| Shivering | 35 (12.54) | 11 (1.47) | <0.0001a |
| Diarrhea | 18 (6.45) | 35 (4.68) | 1.304 |
Characteristics, clinical spectrum and severity of symptomatic COVID-19 infections among vaccinated and unvaccinated individuals.
Values presented as n (%) and chi square test used for the comparison.
represents the significant outcome at p-value <0.05.
We further divided the characteristics and clinical spectrum of participants who had symptomatic COVID-19 infection based on their vaccination status during Wave-II (Table 3) and Wave-III (Table 4). The proportion of symptoms such as fever, fatigue, and shortness of breath was found to be significantly higher among the participants who had symptomatic infection during Wave-II (83.9%; 556/663) and had taken at least one dose of COVID-19 vaccine (Table 3). Among the participants (66.1%; 158/239) who had symptomatic infection during Wave-III, we did not find any difference between the characteristics and clinical symptoms of vaccinated and unvaccinated participants (Table 4).
TABLE 3
| Wave-II | Vaccine type | Vaccinated (N = 116) | Unvaccinated (N = 440) | P-value | |
|---|---|---|---|---|---|
| Covaxin (N = 20) | Covishield (N = 96) | ||||
| Gender | |||||
| Males | 10 (50.0) | 54 (56.25) | 64 (55.17) | 225 (51.14) | 0.440 |
| Females | 10 (50.0) | 42 (43.75) | 52 (44.83) | 215 (48.86) | |
| Median Age (range) | |||||
| 18–40 | 10 (50.0) | 46 (47.92) | 56 (48.28) | 333 (75.68) | <0.001* |
| 41–60 | 10 (50.0) | 35 (36.46) | 45 (38.79) | 94 (21.36) | |
| 61 and above | 0 (0) | 15 (15.63) | 15 (12.93) | 13 (2.95) | |
| Comorbidities present | 7 (35.0) | 26 (27.08) | 33 (28.44) | 93 (21.14) | 0.095 |
| No. of Severe infection/hospitalization | 0 (0) | 6 (6.25) | 6 (5.17) | 23 (5.23) | 0.979 |
| Common Symptoms | |||||
| Fever | 13 (65.0) | 59 (61.46) | 72 (62.07) | 169 (38.41) | <0.001* |
| Loss of taste and smell | 7 (35.0) | 35 (36.46) | 42 (36.21) | 157 (35.68) | 0.916 |
| Fatigue | 1 (5.0) | 25 (26.04) | 26 (22.41) | 65 (14.77) | 0.048* |
| Headache | 8 (40.0) | 27 (28.13) | 35 (30.17) | 120 (27.27) | 0.536 |
| Body ache | 5 (25.0) | 27 (28.13) | 32 (27.59) | 116 (26.36) | 0.790 |
| Shortness of breath | 4 (20.0) | 19 (19.79) | 23 (19.83) | 50 (11.36) | 0.016* |
| Running nose | 2 (10.0) | 6 (6.25) | 8 (6.90) | 29 (6.59) | 0.905 |
| Persistent Cough | 0 (0) | 3 (3.13) | 3 (2.59) | 15 (3.41) | 0.657 |
| Chest pain | 2 (10.0) | 8 (8.33) | 10 (8.62) | 30 (6.82) | 0.505 |
| Hoarse voice | 0 (0) | 2 (2.08) | 2 (1.72) | 10 (2.27) | 0.717 |
| Anxiety | 1 (5.0) | 9 (9.38) | 10 (8.62) | 29 (6.59) | 0.446 |
| Shivering | 1 (5.0) | 1 (1.04) | 2 (1.72) | 11 (2.50) | 0.621 |
| Diarrhea | 0 (0.0) | 8 (8.33) | 8 (6.90) | 20 (4.55) | 0.303 |
Vaccination status and clinical spectrum of COVID-19 among symptomatic individuals during Wave-II (N = 556; 83.9%).
TABLE 4
| Wave-III | Vaccine type | Vaccinated (N = 145) | Unvaccinated (N = 13) | P-value | |
|---|---|---|---|---|---|
| Covaxin (N = 13) | Covishield (N = 132) | ||||
| Gender | |||||
| Males | 7 (53.85) | 71 (53.78) | 78 (53.79) | 7 (53.85) | 0.736 |
| Females | 6 (46.15) | 61 (46.21) | 67 (46.21) | 6 (46.15) | |
| Median Age (range) | |||||
| 18–40 | 11 (84.62) | 81 (61.36) | 92 (63.44) | 9 (69.23) | 0.422 |
| 41–60 | 1 (7.69) | 31 (23.48) | 32 (22.07) | 4 (30.77) | |
| 61 and above | 1 (7.69) | 20 (15.15) | 21 (14.48) | 0 (0) | |
| Comorbidities present | 3 (23.08) | 15 (11.36) | 18 (12.41) | 2 (15.38) | 0.707 |
| Mild | 7 (53.85) | 100 (75.76) | 107 (73.79) | 9 (69.23) | 0.592 |
| Moderate | 5 (38.46) | 24 (18.18) | 29 (20.0) | 3 (23.08) | 0.680 |
| No. of Severe infection/hospitalization | 1 (7.69) | 8 (6.06) | 9 (6.21) | 1 (7.69) | 0.386 |
| Common Symptoms | |||||
| Fever | 11 (84.62) | 63 (47.73) | 74 (51.03) | 5 (38.46) | 0.533 |
| Loss of taste and smell | 0 (0) | 46 (34.85) | 46 (31.72) | 1 (7.69) | 0.089 |
| Fatigue | 7 (53.85) | 30 (22.73) | 37 (25.52) | 4 (30.77) | 0.555 |
| Headache | 4 (30.77) | 29 (21.97) | 33 (22.76) | 3 (23.08) | 0.859 |
| Body ache | 6 (46.15) | 33 (25.0) | 39 (26.89) | 2 (15.38) | 0.439 |
| Shortness of breath | 1 (7.69) | 15 (11.36) | 16 (11.03) | 1 (7.69) | 0.772 |
| Running nose | 6 (46.15) | 17 (12.88) | 23 (15.86) | 4 (30.77) | 0.123 |
| Persistent Cough | 7 (53.85) | 13 (9.85) | 20 (13.79) | 2 (15.38) | 0.782 |
| Chest pain | 1 (7.69) | 2 (1.52) | 3 (2.07) | 1 (7.69) | 0.186 |
| Hoarse voice | 2 (15.38) | 8 (6.06) | 10 (6.90) | 2 (15.38) | 0.221 |
| Anxiety | 0 | 9 (6.82) | 9 (6.21) | 0 | 0.374 |
| Shivering | 5 (38.46) | 27 (20.45) | 32 (22.07) | 2 (15.38) | 0.663 |
| Diarrhea | 1 (7.69) | 6 (4.55) | 7 (4.83) | 1 (7.69) | 0.596 |
Vaccination status and clinical spectrum of COVID-19 among symptomatic individuals during Wave-III (N = 158; 66.1%).
Breakthrough infections across different waves
Out of 1297 SARS-CoV-2 infected individuals, 28% (364/1,297) contracted SARS-CoV-2 post-vaccination, where breakthrough infections relative to the total number of Covishield and Covaxin recipients were 16.33% (318/1947) and 9.97% (46/461), respectively (Covishield-vaccinated, n = 318 and Covaxin-vaccinated, n = 46). The post-vaccination infection rate was found to have increased from the Wave-II to Wave-III among the participants who had received the Covishield vaccine (Figure 2B).
We compared the breakthrough cases vaccine-wise and investigated other parameters (Supplementary Table S3). The Covishield-vaccinated participants showed the highest breakthrough infections during the Wave-III in comparison to Covaxin-vaccinated participants, although the incidence of comorbidities was low among Covishield-vaccinated participants (Supplementary Table S3). We did not observe any other difference among the other parameters based on vaccine type.
Characterizing post-COVID-19 sequelae
We also investigated the post-COVID-19 sequelae among the participants who were infected with COVID-19. A total of 463 individuals responded to the question if they experienced long COVID-19 symptoms. Around 192 participants responded “Yes” and reported the post-COVID-19 sequelae experienced by them. We investigated the vaccination status among the participants who experienced post-COVID-19 sequelae. Among the participants who experienced post-COVID-19 sequelae 96.9% (186/192) were vaccinated and 3.1% (6/192) were unvaccinated (Supplementary Table S4). The frequency of post-COVID-19 sequelae was found to be the highest during the Wave-III, where most participants got COVID-19 infections after vaccination (Figure 3A). The frequency of Covishield-vaccinated participants experienced the highest post-COVID-19 sequelae in comparison to Covaxin vaccinated during the second and third waves (Figure 3B). However, a non-significant difference in the post-COVID-19 sequelae was observed in terms of the total number of Covishield and Covaxin recipients with rates of 7.96% (155/1947) and 6.72% (31/461), respectively. The most prevalent symptom was fatigue, followed by loss of taste/smell, extreme hair fall, and joint pain/chest pain. However, symptoms like menstrual irregularities and brain fog were less frequent and can be only attributed to participants who were Covishield vaccinated (Figure 3C).
FIGURE 3
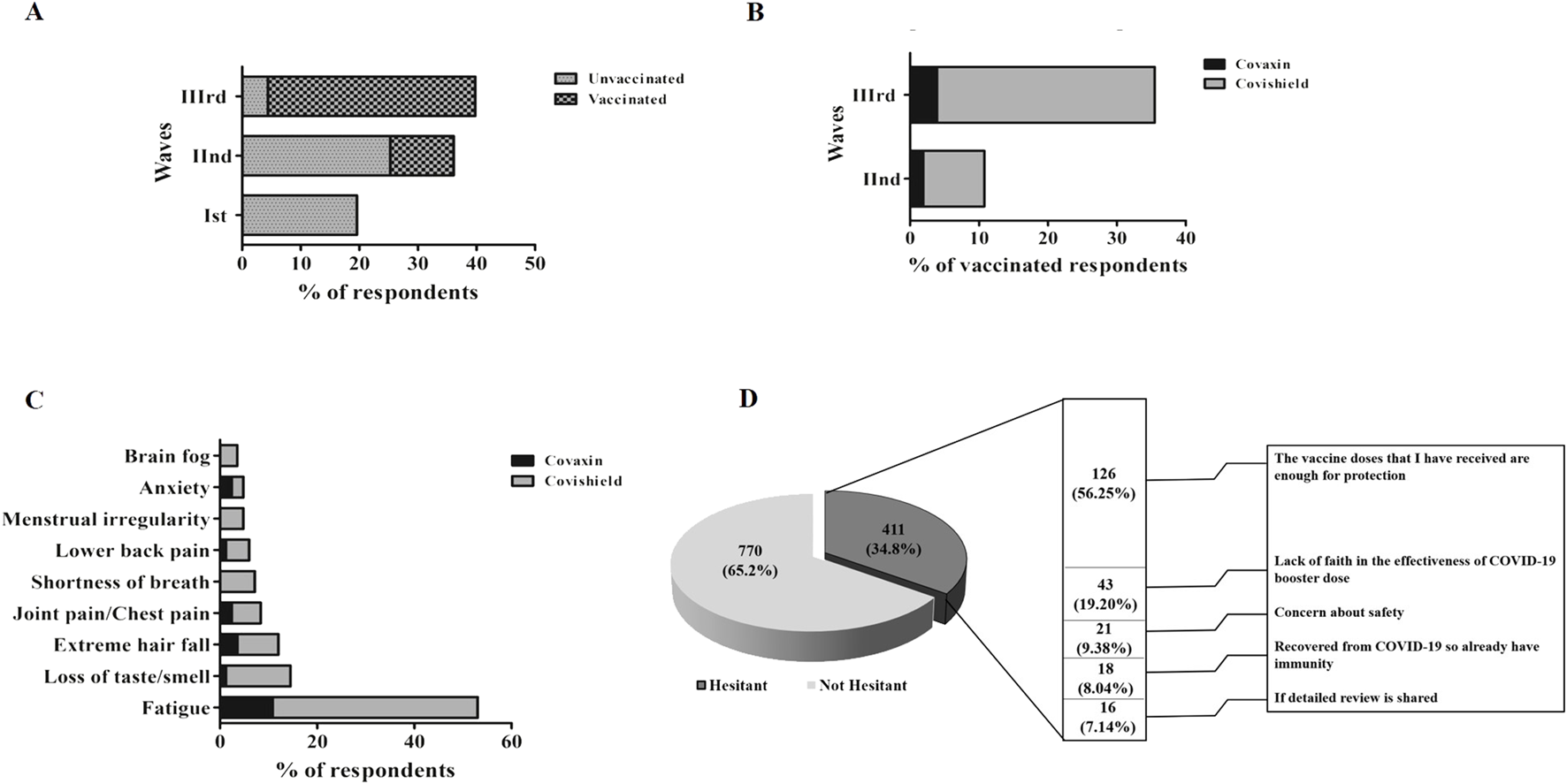
(A) Distribution of post-COVID-19 sequelae across three waves. (B) Vaccine-wise distribution of post-COVID-19 sequelae among three waves. (C) Vaccine-wise distribution of the clinical spectrum of post-COVID-19 sequelae post-vaccination. (D) Barriers to accept the COVID-19 booster dose.
The highest proportion (66%) of individuals experiencing post-COVID-19 sequelae belonged to the age group 18–40 years (Supplementary Table S4) A total of 59.4% of females reported post-COVID-19 sequelae in comparison to 40.6% of males. Most individuals who developed post-COVID-19 complications were infected either during the Wave-II or Wave-III (Supplementary Table S4). Notably, we also investigated the impact of vaccination on long COVID-19 sequelae, particularly among those who had infection before vaccination. Among the participants developing long COVID-19 after the Wave-I (16%) and Wave-II (20%) reported receiving vaccination after recovery from COVID-19 (Supplementary Table S4). A high proportion (73%) of the participants with post-COVID-19 sequelae had co-morbidities (Supplementary Table S4).
Willingness to take COVID-19 booster dose
As we observed a shift in the frequency of COVID-19 symptoms among vaccinated individuals, we further investigated about the willingness of the individuals to receive a COVID-19 booster dose. A total of 1,181 individuals responded to the question, if they were willing to take the COVID-19 booster dose. Out of 1,181, 411 (34.8%) responded “No” and 770 (65.2%) responded “Yes,” resulting in 38% COVID-19 booster dose hesitancy. A high proportion (55.1%) of females and young age group participants (83.2%) were observed to be more hesitant than males (44.9%) and older age group participants.
As shown in Figure 3D, the most common reason cited for COVID-19 booster dose hesitancy among participants was the belief of sufficiency of initial 2 doses (56.3%). The concern in distant second place was the lack of faith in the effectiveness of the COVID-19 booster dose (19.2%).
Discussion
The study performs a comprehensive comparative analysis of symptoms, severity, and breakthrough infections after vaccination with either the Covishield or Covaxin vaccines across three waves of COVID-19 in India. Our study highlights that the proportion of asymptomatic COVID-19 infection increased from the Wave-I to Wave-III (∼33%). However, the impact of vaccination and vaccine types (Covaxin and Covishield) on the prevalence of asymptomatic infections has not been studied previously. An increased proportion of asymptomatic infection was observed among the participants who were vaccinated with Covishield or Covaxin vaccine in comparison to the symptomatic infection, confirming the role of vaccination in ameliorating the severity of COVID-19 infection. Our results corroborate the previous study highlighting the fact that the performance of the COVID-19 vaccine is driven by its ability to result in asymptomatic infections or mild infections leading to optimization of reduction in hospitalization (Aguiar et al., 2022). The reason behind increased symptomatic cases over asymptomatic cases within the Wave-III could be attributed to the exclusion of self-reported asymptomatic cases who circumvented RT-PCR testing as well as uncertain cases who might be COVID-19 affected but were not sure of infection in the absence of symptoms and a confirmed test report because COVID-19 testing was not required for asymptomatic infections as per MoHFW (Ministry of Health and Family Welfare) guidelines (Revised guidelines for Home Isolation, 2022). Also, asymptomatic cases posed a higher challenge in predicting COVID-19 cases due to weaker forms of prevalent Omicron strain in comparison to other variants of concern (VOCs). This could be the reason for a lower reporting of asymptomatic cases within the Wave-III leading to a higher percentage of symptomatic cases.
Despite the Omicron variant being the most divergent and having a high transmission rate, robust binding affinity, immune escape, and antibody resistance (Saxena et al., 2022), we did not find a high incidence of COVID-19 infection during the Wave-III in comparison to the Wave-I and Wave-II, suggesting that mild or asymptomatic infection during the Wave-III may be the prevalent factor associated with under-diagnosis of COVID-19. Further, on studying the clinical characteristics of participants, we found a significantly high prevalence (∼16.3%) of COVID-19 in rural areas during the Wave-III in comparison to the two preceding waves. Also, a higher proportion of individuals in rural areas developed COVID-19 during the Wave-III, which was witnessed in clinical practice. The high diffusion of infections in rural areas may be due to the high transmission rate of the Omicron variant along with unlocking at both national and regional levels, which leads to migration and dissemination of infection from urban to rural areas (Ma et al., 2021). Notably, the frequency of occurrence of fever and persistent cough reduced significantly in the Wave-III as compared to the earlier waves. The results are consistent with the study performed on children and youth in Hong Kong, where they found a smaller number of COVID-19 patients with fever and cough who were infected during the second and third waves in comparison to the Wave-I (Chua et al., 2021). Moreover, consistent with our results, loss of smell, chest pain, and shortness of breath were known to be the rare symptoms of the Omicron variant (Iram et al., 2022). The frequency of the hoarse voice, cold/running nose, and shivering was found to be significantly increased in the Wave-III as compared to the earlier waves. These differences in the clinical symptoms of infected individuals might be due to the predominance of different variants in circulation across the three waves.
Furthermore, on investigating the characteristics and clinical spectrum of symptomatic COVID-19 infection among vaccinated and unvaccinated participants, we found an increased proportion of a few symptoms such as fever, fatigue, shortness of breath, persistent cough, hoarse voice, and shivering among vaccinated participants in comparison to unvaccinated. However, on bifurcating the data wave-wise (Wave-II and Wave-III), we found that the proportion of symptoms such as fever, fatigue, and shortness of breath was significantly higher among the vaccinated participants who contracted the disease during Wave-II but not during Wave-III implicating that COVID-19 infected participants might develop a high proportion of COVID-19 symptoms during Wave-II despite being vaccinated but this proportion of symptoms was insignificant in Wave-III, which might be due to a weaker form of the prevalent strain i.e., the Omicron variant. The reason for increased symptoms in vaccinated individuals during the Wave-II could be due to a highly infectious double mutant variant (B.1.617 lineage) that might have evaded the immune defense.
We observed increased breakthrough infections during the Wave-II to Wave-III, in contrast to a previous study carried out by Arora et al. (7.91%) (Arora et al., 2022). In addition, we found a high COVID-19 infection rate post-vaccination during the Wave-III in comparison to the Wave-II, suggesting that increased breakthrough infections might be due to the Omicron variant rather than the Delta variant. Our conclusions are further supported by a recent study that explains hybrid immune damping. This study identifies considerable differential subversion of immune recognition and differential regulation through immunological imprinting as the root causes of greater breakthrough infections and frequent reinfections during the Wave-III (Reynolds et al., 2022). This could avert severe infections in the Wave-III, resulting primarily in asymptomatic infections. Similar to the previous study by Kaur et al. (2022a), we have observed a high rate of COVID-19 breakthrough infections among younger age groups. This elevated risk may be the result of altered social behavior, such as more interactions with young people who have not received vaccinations, more interactions in social settings, and more interactions at work. The most common manifestations of post-COVID-19 sequelae were observed to be systemic (fatigue and joint pain), neuropsychiatric (anxiety, brain fog, loss of smell/taste), respiratory (shortness of breath, chest pain), extreme hair loss, and menstrual irregularities. Several studies also reported post-COVID-19 symptoms such as anxiety, breathlessness, chest pain, and hair loss (Taquet et al., 2021; Huang et al., 2021). A recent study showed the persistence of post-COVID-19 symptoms is due to a delay in the resolution of the inflammatory response to COVID-19 infection (Phetsouphanh et al., 2022). Since the inflammatory response and autoimmune response to infection have been reported in severe infection (Wang et al., 2021), we further investigated the severity of participants reporting post-COVID-19 sequelae in our study. We found that 29% of participants had severe infection; however, the remaining participants had mild (43%) or moderate (28%) infection, speculating that persistent post-COVID-19 manifestations are independent of the severity of infection. Nearly 36.5% (70/192) of individuals with long COVID-19 had a history of receiving any vaccine after recovery from COVID-19. Previously, the vaccine received after recovery from COVID-19 has been shown as an independent risk factor for long COVID-19 highlighting the need to be vigilant in this subgroup (Kaur et al., 2022b). In addition, recent studies revealed that vaccination ameliorates reinfection and sequelae following reinfection (Bahadir et al., 2023; Català et al., 2024). However, further investigation is required to comprehend the mechanisms underlying COVID-19 sequelae.
In this study, a significant proportion of young and female participants were hesitant to receive the COVID-19 booster doses among a total of 34.8% of participants who were reluctant to receive a booster dose. The booster dose hesitancy (34.8%) was found significantly higher in comparison to primary vaccine hesitancy in both adults and children as shown in the previous studies (Jetly et al., 2022; Bendau et al., 2021; Bhardwaj et al., 2024; Goruntla et al., 2023). The possible causes could be the spread of erroneous information related to female infertility and COVID-19 vaccine on social media (Morris, 2021). As many participants were reluctant to take the booster dose because of safety issues, the long-term safety of COVID-19 vaccines and the effectiveness of the COVID-19 booster dose should be studied, and data provided in the public domain to help resolve the vaccine hesitancy.
Conclusion
The increased symptoms in vaccinated individuals during the Wave-II highlighted the probable role of antibody dependent enhancement. A significant percentage (36.5%) of individuals with long COVID-19 were found to have a history of receiving vaccine after natural COVID-19 infection. The fact that the vaccination received after COVID-19 infection has been demonstrated to be a risk factor for long COVID-19 highlights the requirement for caution in this specific subgroup. We believe that these findings will aid in the development of an effective public health strategy based on targeted interventions and resource allocation. A comprehensive risk communication can improve public knowledge and acceptance of vaccination. The study may aid in data-driven preparedness for future epidemics, resulting in better response measures. Together, these advantages lead to a healthier and more resilient society.
Limitation of the study
Around 73% of the participants in the present study belonged to the age group 18–40 years. It has been apparent that during COVID times, this age group had minimum manifestation and severity of symptoms. Also, the symptoms given in the present study are on the recall responses of individuals. Although our study clearly demonstrated the high effectiveness of two doses of vaccine against the low incidence and mild or asymptomatic type of SARS-CoV-2 infections in comparison to a single dose; however, we could not estimate the severity of SARS-CoV-2 infection among the participants who have received either a double or single dose of vaccines due to the small sample size. Similarly, we did not find any difference among the hospitalized cases across three waves due to the small sample size. Further, our analysis showed high rates of asymptomatic infection and breakthrough infections among those who had been administered Covishield in comparison to Covaxin; however, this data is constrained because around 80% of the participants in our study were administered the Covishield vaccine.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Dr. B.R. Ambedkar Centre of Biomedical Research, University of Delhi, Delhi. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JT, DS, SJ, PB, and SY designed the survey form. JT, DS, SJ, PB, and SY collected the epidemiological data. PB, JT, RK, and KN analysed the data. PB, JT, DS, SY, RK, and SJ drafted the manuscript. All authors read the manuscript and approved it. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to acknowledge all the participants and volunteers for collecting offline data from door to door. We thank Prof. Savita Roy (Principal, Daulat Ram College, University of Delhi) and Prof. Ravi Toteja (Principal, Acharya Narendra Dev College, University of Delhi) and for their logistic support and cooperation. We would like to thanks Council of Scientific and Industrial Research (CSIR) for research support.
Conflict of interest
Author KN was employed by Ipca Laboratories (India).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/av.2024.13536/full#supplementary-material
References
1
AccorsiE. K.BrittonA.Fleming-DutraK. E.SmithZ. R.ShangN.DeradoG.et al (2022). Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-COV-2 Omicron and Delta variants. JAMA327, 639. 10.1001/jama.2022.0470
2
AguiarM.Van-DierdonckJ. B.MarJ.StollenwerkN. (2022). The role of mild and asymptomatic infections on COVID-19 vaccines performance: a modeling study. J. Adv. Re39, 157–166. 10.1016/j.jare.2021.10.012
3
AroraG.TanejaJ.BhardwajP.GoyalS.NaiduK.YadavS. K.et al (2022). Adverse events and breakthrough infections associated with COVID-19 vaccination in the Indian population. J. Med. Virol.94 (7), 3147–3154. 10.1002/jmv.27708
4
BahadirS.KabacaogluE.MemisK. B.HasanH. I.AydinS. (2023). The effects of vaccines on the sequelae rates of recurrent infections and the severity of pulmonary COVID-19 infection by imaging. Vaccines (Basel)11 (8), 1321. 10.3390/vaccines11081321
5
BendauA.PlagJ.PetzoldM. B.StröhleA. (2021). COVID-19 vaccine hesitancy and related fears and anxiety. Int. Immunopharmacol.97, 107724. 10.1016/j.intimp.2021.107724
6
BhardwajP.YadavS.JetlyS.SalujaD.TanejaJ. (2024). Unveiling parental perspectives: COVID-19 vaccination for children in India. J. Fam. Med. Prim. Care13 (4), 1481–1487. 10.4103/jfmpc.jfmpc_1485_23
7
CatalàM.Mercadé-BesoraN.KoldeR.TrinhN. T. H.RoelE.BurnE.et al (2024). The effectiveness of COVID-19 vaccines to prevent long COVID symptoms: Staggered cohort study of data from the UK, Spain, and Estonia. Lancet Respir. Med.12 (3), 225–236. 10.1016/s2213-2600(23)00414-9
8
CDCVariants of the virus. 2022Available at: https://www.cdc.gov/coronavirus/2019-ncov/variants/index.html. (Accessed 27 October 2022)
9
ChuaG. T.WongJ. S. C.LamI.HoP. P. K.ChanW. H.YauF. Y. S.et al (2021). Clinical characteristics and transmission of COVID-19 in children and youths during 3 waves of outbreaks in Hong Kong. JAMA Netw. Open4 (5), e218824. 10.1001/jamanetworkopen.2021.8824
10
CrossR. (2021). Omicron puts scientists on red alert. ChemEng News99, 44.
11
EllaR.ReddyS.BlackwelderW.PotdarV.YadavP.SarangiV.et al (2021). Efficacy, safety, and a lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): a double-blind, randomised, controlled phase 3 trial. bioRxiv. 10.1016/S0140-6736(21)02000-6
12
El-ShabasyR. M.NayelM. A.TaherM. M.AbdelmonemR.ShoueirK. R.KenawyE. R. (2022). Three waves changes, new variant strains, and vaccination effect against COVID-19 pandemic. Int. J. Biol. Macromol.204, 161–168. 10.1016/j.ijbiomac.2022.01.118
13
GoruntlaN.AyishaM. U.SreeramM. (2023). Predictors of parents' willingness to vaccinate their children against COVID-19 in India: a web-based cross-sectional survey. Health Serv. Res. Manag. Epidemiol.10, 23333928231175798. 10.1177/23333928231175798
14
HuangC.HuangL.WangY.LiX.RenL.GuX.et al (2021). Retracted: 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet397 (10270), 220–232. 10.1016/s0140-6736(20)32656-8
15
IramF.VatsA.RajuA.ChandoliaB.GahlotM.HariharanN.et al (2022). Advent of omicron: an attempt to decode the third wave covid variant. Br. J. Healthc. Med. Res.9 (3), 59–74. 10.14738/jbemi.93.12213
16
JetlyS.BhardwajP.AroraG.SalujaD.YadavS. K.NaiduK. P.et al (2022). Hesitancy and acceptance of COVID-19 vaccination amidst the second wave of pandemic in India: a general population study. Asia-Pacific J. public health34 (4), 446–449. 10.1177/10105395221077062
17
KapoorM.PandaP. K. (2022). India's second COVID wave: how is it different from the first wave?Int. J. Infect. Dis.116, S50–S9712. 10.1016/j.ijid.2021.12.121
18
KaurU.BalaS.JoshiA.ReddyN. T. S.JapurC.ChauhanM.et al (2022b). Persistent health issues, adverse events, and effectiveness of vaccines during the second wave of COVID-19: a cohort study from a tertiary hospital in north India. Vaccines (Basel)10 (7), 1153. 10.3390/vaccines10071153
19
KaurU.BalaS.OjhaB.JaiswalS.KansalS.ChakrabartiS. S. (2022a). Occurrence of COVID-19 in priority groups receiving ChAdOx1 nCoV-19 coronavirus vaccine (recombinant): a preliminary analysis from north India. J. Med. Virol.94 (1), 407–412. 10.1002/jmv.27320
20
KumarG.MukherjeeA.TurukA.BhallaA.TalukdarA.ShivnitwarS. K.et al (2022). Characterizing the third wave of COVID-19: an analysis from the national clinical registry of COVID-19. Indian J. Med. Res.478–484. 10.4103/ijmr.ijmr_276_22
21
MaQ.LiuJ.LiuQ.KangL.LiuR.JingW.et al (2021). Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw. Open4 (12), e2137257. 10.1001/jamanetworkopen.2021.37257
22
MohapatraR. K.KandiV.VermaS.DhamaK. (2022). Challenges of the omicron (B.1.1.529) variant and its lineages: A global perspective. Chembiochem23, e202200059. 10.1002/cbic.202200059
23
MorrisR. S. (2021). SARS-CoV-2 spike protein seropositivity from vaccination or infection does not cause sterility. F. S Rep.2 (3), 253–255. 10.1016/j.xfre.2021.05.010
24
PeregoE.CallardF.StrasL.Melville-JóhannessonB.PopeR.AlwanN. A. (2020). Why the patient-made term “long covid” is needed. Wellcome Open Res.5, 224. 10.12688/wellcomeopenres.16307.1
25
PhetsouphanhC.DarleyD. R.WilsonD. B.HoweA.MunierC. M. L.PatelS. K.et al (2022). Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol.23 (2), 210–216. 10.1038/s41590-021-01113-x
26
RamasamyM. N.MinassianA. M.EwerK. J.FlaxmanA. L.FolegattiP. M.OwensD. R.et al (2021). Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet396, 1979–1993. 10.1016/s0140-6736(20)32466-1
27
Revised guidelines for Home Isolation of mild/asymptomatic COVID-19 cases. 2022. Available at: https://www.mohfw.gov.in/pdf/RevisedHomeIsolationGuidelines05012022.pdf
28
ReynoldsC. J.PadeC.GibbonsJ. M.OtterA. D.LinK. M.Muñoz SandovalD.et al (2022). Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science377 (6603), eabq1841. 10.1126/science.abq1841
29
SamarasekeraU. (2021). India grapples with second wave of COVID-19. Lancet Microbe2 (6), E238. 10.1016/s2666-5247(21)00123-3
30
SaxenaS. K.KumarS.AnsariS.PaweskaJ. T.MauryaV. K.TripathiA. K.et al (2022). Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) variant of concern and its global perspective. J. Med. Virol.94 (4), 1738–1744. 10.1002/jmv.27524
31
SharmaA.TiwariS.DebM. K.MartyJ. L. (2020). Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents56 (2), 106054. 10.1016/j.ijantimicag.2020.106054
32
TaquetM.LucianoS.GeddesJ. R.HarrisonP. J. (2021). Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry8 (2), 130–140. 10.1016/s2215-0366(20)30462-4
33
WangE. Y.MaoT.KleinJ.DaiY.HuckJ. D.JaycoxJ. R.et al (2021). Diverse functional autoantibodies in patients with COVID-19. Nature595 (7866), 283–288. 10.1038/s41586-021-03631-y
34
World Health Organisation (2023). Available at: https://www.who.int/docs/default-source/coronaviruse/21112023_ba.2.86_ire.pdf?sfvrsn=8876def1_3.
35
ZhangY. Z.HolmesE. C. (2020). A genomic perspective on the origin and emergence of SARS-CoV-2. Cell181, 223–227. 10.1016/j.cell.2020.03.035
36
ZhouY.ZhiH.TengY. (2023). The outbreak of SARS-CoV-2 Omicron lineages, immune escape, and vaccine effectivity. J. Med. Virol.95 (1), e28138. 10.1002/jmv.28138
37
ZhuZ.LianX.SuX.WuW.MarraroG. A.ZengY. (2020). From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res.21, 224. 10.1186/s12931-020-01479-w
38
ZiauddeenN.GurdasaniD.O'HaraM. E.HastieC.RoderickP.YaoG.et al (2022). Characteristics and impact of long covid: findings from an online survey. PloS one17 (3), e0264331. 10.1371/journal.pone.0264331
Summary
Keywords
breakthrough infections, COVID-19, infection severity, vaccination, COVID-19 symptoms
Citation
Bhardwaj P, Jetly S, Yadav S, Kaushik R, Naidu K, Saluja D and Taneja J (2024) Comparative analyses of symptoms, severity, and breakthrough infections in vaccinated and unvaccinated individuals during three waves of COVID-19 in India. Acta Virol. 68:13536. doi: 10.3389/av.2024.13536
Received
15 July 2024
Accepted
08 November 2024
Published
05 December 2024
Volume
68 - 2024
Edited by
Žofia Rádiková, Slovak Academy of Sciences, Slovakia
Reviewed by
Amrendra Kumar, Vanderbilt University Medical Center, United States
Pujarini Dash, Regional Medical Research Center (ICMR), India
Updates
Copyright
© 2024 Bhardwaj, Jetly, Yadav, Kaushik, Naidu, Saluja and Taneja.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jyoti Taneja, jyoti4arora@gmail.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.