Abstract
Disruption of the female genital microbiome is associated with several pregnancy complications, including miscarriage, preterm onset of labour, and tubal pregnancy. Ectopic pregnancy is a known cause of maternal morbidity and mortality, but early diagnosis and treatment of ectopic pregnancy remain a challenge. Despite growing established associations between genital microbiome and female reproductive health, few studies have specifically focused on its link with ectopic pregnancy. Therefore, the current review aims to provide a comprehensive account of the female genital microbiome in healthy and fertile women compared to those in ectopic pregnancy and its associated risk factors. The microbial diversity from various sites of the female genital tract was explored for a reliable proxy of female reproductive health in sequencing-based ectopic pregnancy research. Our report confirmed the predominance of Lactobacillus in the vagina and the cervix among healthy women. The relative abundance decreased in the vaginal and cervical microbiome in the disease state. In contrast, there were inconsistent findings on the uterine microbiome across studies. Additionally, we explore a spectrum of opportunities to enhance our understanding of the female genital tract microbiome and reproductive conditions. In conclusion, this study identifies gaps within the field and emphasises the need for visionary solutions in metagenomic tools for the early detection of ectopic pregnancy and other gynaecological diseases.
Introduction
The female genital tract can be separated into the upper genital tract, which comprises the ovaries, fallopian tubes, endometrium, and cervix, and the lower genital tract, which is made up of the vulva and the vagina [1]. Generally, it has been agreed upon that the vagina is colonised by a wide range of bacteria but is physiologically dominated by Lactobacillus. [2] In contrast, the fallopian tubes and endometrium have classically been described as sterile sites, protected by cervical mucus, which acts as a barrier to the ascent of bacteria into the uterus [3]. However, this notion has been challenged, as it has been shown that particles can be transported from the vagina to the upper genital tract during the follicular and luteal phases of the menstrual cycle [4].
Prior to 2007, characterisation of the female genital tract was mostly done by conventional culture methods. However, this was gradually taken over by next-generation sequencing (NGS), such as 16S rRNA gene sequencing [5]. The 16S rRNA gene, also known as 16S rDNA, is the part of the DNA most commonly used for the purpose of taxonomic classification of bacteria. This method works well for samples contaminated by host DNA and low biomass samples, such as the upper genital tract [6]. Although the majority of primary research studies characterising female reproductive tract microbiome focused on the vagina due to its acceptability and ease of sampling [2, 7, 8], a number of studies investigated the cervical microbiome, with scant and fragmented evidence on the microbiome above the cervix [9].
Due to various limitations, little research has been done on the microbiome of the female genital tract in ectopic pregnancy. Researchers postulate that endometrial microbiota may play a role in the pathogenesis of ectopic pregnancy [10]. With existing knowledge, imbalances of endometrial microbiota have been associated with endometriosis, infertility, and recurrent pregnancy loss [5, 11]. Some widely explored risk factors for ectopic pregnancy include recurrent ectopic pregnancy [12], pelvic inflammatory disease [13], endometriosis, and adenomyosis [14]. In this review, we explored the similarities in these conditions and or risk factors associated with ectopic pregnancy, the changes in relative abundances of the microbiome, and the changes in diversity compared to the microbiome of healthy, fertile women. With gathered evidence, reliable proxies for potential early diagnosis and disease management in ectopic pregnancy are also discussed.
Diversity of the Female Genital Tract Microbiome in Healthy Women
PubMed, Scopus and Ovid MEDLINE databases were used and manually screened by title, abstract, and full text for relevance at the same time, noting the inclusion and exclusion criteria. Only women of reproductive age were recruited whilst studies that recruited women who used hormonal contraceptives were excluded. Of the 31 studies selected for this review (Supplementary Material), 15 were from China, 4 from Italy, 2 from Japan, and 1 each from Australia, Spain, Germany, Turkey, Puerto Rico, Korea, United States, Taiwan, Sweden, and Thailand. The sample sizes ranged from 5 to 160 participants. Some studies investigated the microbiome of more than one genital site. Two studies analysed the microbiome of the fallopian tube, three looked at the endometrium, eight focused on the cervix, and twenty-four studies described the microbiome of the vagina. Some studies provided the mean relative abundance in percentage of the top 10-20 taxa, while some only arranged the taxa identified in order of decreasing abundance. Table 1 summarises the findings from the 31 studies included. Healthy, fertile controls from studies that characterised the microbiome in women with reproductive health conditions and in infertile women were also included, provided they were not pregnant, not using any hormonal contraception, and were not pre-menopausal. At the level of phyla, the microbiome of the female genital tract in healthy, fertile women is composed mainly of Actinobacteria, Bacteroidetes, Firmicutes, Fusobacteria, and Proteobacteria, with few studies identifying Tenericutes. Acidobacteria, Chlamydiae, Chlorofexi, Planctomycetes, and Verrucomicrobia were only identified in one study. The microbiome is also not consistent throughout the female genital tract, with variations between the fallopian tube, endometrium, cervix, and vagina.
TABLE 1
| Study | Sample size | Country | Sample type | Sequencing techniques (Target region) | Major taxa (mean relative abundance, %) | Ref. |
|---|---|---|---|---|---|---|
| Fallopian tube | ||||||
| Pelzer et al. (2018) | 8 | Australia | Fallopian tube dissection | 454 pyrosequencing (V5-V8) | Staphylococcus | [15] |
| Escherichia | ||||||
| Pseudomonas | ||||||
| Zhou et al. (2019) | 25 | China | Fallopian tube fimbria tissue | Illumina Miseq (V3-V4) | Proteobacteria | [16] |
| Firmicutes | ||||||
| Bacteroidetes | ||||||
| Actinobacteria | ||||||
| Chlorofexi | ||||||
| Acidobacteria | ||||||
| Fusobacteria | ||||||
| Endometrium | ||||||
| Fang et al. (2016) | 10 | China | Endometrial swabs | Illumina Miseq (V4) | Enterobacter (33.41%) | [17] |
| Pseudomonas (23.56%) | ||||||
| Lactobacillus (6.23%) | ||||||
| Desulfosporosinus (4.33%) | ||||||
| Ralstonia (4.26%) | ||||||
| Gardnerella (3.55%) | ||||||
| Cupriavidus (0.92%) | ||||||
| Prevotella (0.83%) | ||||||
| Thalassospira (0.79%) | ||||||
| Sphingomonas (0.77%) | ||||||
| Vibrio (0.74%) | ||||||
| Streptococcus (0.59%) | ||||||
| Atopobium (0.58%) | ||||||
| Bifidobacterium (0.58%) | ||||||
| Klebsiella (0.53%) | ||||||
| Megasphaera (0.52%) | ||||||
| Pelomonas (0.51%) | ||||||
| Alteromonas (0.45%) | ||||||
| Marinobacter (0.24%) | ||||||
| Erythrobacter (0.22%) | ||||||
| Veillonella (0.21%) | ||||||
| Muricauda (0.19%) | ||||||
| Methylobacterium (0.19%) | ||||||
| Escherichia (0.18%) | ||||||
| Bacillus (0.17%) | ||||||
| Mobiluncus (0.16%) | ||||||
| Singulisphaera (0.16%) | ||||||
| Tolumonas (0.15%) | ||||||
| Dialister (0.14%) | ||||||
| Thiothrix (0.14%) | ||||||
| Sneathia (0.13%) | ||||||
| Halomonas (0.11%) | ||||||
| Gemmata (0.11%) | ||||||
| Acinetobacter (0.10%) | ||||||
| Aquabacterium (0.10%) | ||||||
| Simkania (0.10%) | ||||||
| Moreno et al. (2016) | 44 | Spain | Endometrial fluid (aspirate) | 454 pyrosequencing (V3-V5) | Lactobacillus (71.70%) | [18] |
| Gardnerella (12.60%) | ||||||
| Bifidobacterium (3.70%) | ||||||
| Streptococcus (3.20%) | ||||||
| Prevotella (0.87%) | ||||||
| Kyono et al. (2018) | 15 | Japan | Endometrial fluid (aspirate) | Illumina MiSeq (V4) | Lactobacillus (99.50%) | [19] |
| Cervix | ||||||
| Filardo et al. (2017) | 7 | Italy | Endo-cervical swab | Illumina MiSeq (V3-V4) | Lactobacillus (96.2%) | [20] |
| Gardnerella | ||||||
| Atopobium | ||||||
| Bifidobacterium | ||||||
| Di Pietro et al. (2018) | 7 | Italy | Endo-cervical swab | Illumina MiSeq (V3-V4) | Lactobacillus (96%) | [21] |
| Gardnerella | ||||||
| Atopobium | ||||||
| Bifidobacterium | ||||||
| Graspeuntner et al. (2018) | 89 | Germany | Cervical swab | Illumina MiSeq (V3-V4) | Lactobacillus (78.34%) | [22] |
| Gardnerella (5.43%) | ||||||
| Prevotella (3.02%) | ||||||
| Bifidobacterium (2.45%) | ||||||
| Streptococcus (1.75%) | ||||||
| Enterobacteriaceae, unclassified (1.70%) | ||||||
| Atopobium (1.61%) | ||||||
| Aerococcus (0.72%) | ||||||
| Dialister (0.59%) | ||||||
| Sneathia (0.56%) | ||||||
| Veillonella (0.56%) | ||||||
| Porphyromonas (0.26%) | ||||||
| Clostridiales, unclassified (0.12%) | ||||||
| Ata et al. (2019) | 14 | Turkey | Endocervical swab | Illumina MiSeq (V3-V4) | Lactobacillus | [23] |
| Gardnerella | ||||||
| Prevotella | ||||||
| Atopobium | ||||||
| Dialister | ||||||
| Chorna et al. (2020) | 8 | Puerto Rico | Cervical swab | Not specified | Lactobacillus | [24] |
| Sneathia | ||||||
| Prevotella | ||||||
| Gardnerella | ||||||
| Atopobium | ||||||
| Shuttleworthia | ||||||
| Tu et al. (2020) | 50 | China | Cervical canal swabs | Illumina MiSeq (V3-V4) | Lactobacillus | [25] |
| Gardnerella | ||||||
| Atopobium | ||||||
| Sneathia | ||||||
| Ureaplasma | ||||||
| Wei et al. (2020) | 14 | China | Cervical mucus | Ion Torrent PGM (V4-V5) | Lactobacillus (64.3%) | [26] |
| Qingqing et al. (2021) | 5 | China | Not specified | Ion S5 ™ XL (V4) | Lactobacillus (90.01%) | [27] |
| Vagina | ||||||
| Fang et al. (2016) | 10 | China | Vaginal swab | Illumina Miseq (V4) | Lactobacillus (60.93%) | [17] |
| Gardnerella (15.30%) | ||||||
| Prevotella (6.28%) | ||||||
| Enterobacter (3.27%) | ||||||
| Pseudomonas (2.44%) | ||||||
| Atopobium (1.81%) | ||||||
| Streptococcus (1.32%) | ||||||
| Megasphaera (1.20%) | ||||||
| Bifidobacterium (0.97%) | ||||||
| Sneathia (0.55%) | ||||||
| Desulfosporosinus (0.40%) | ||||||
| Dialister (0.38%) | ||||||
| Veillonella (0.34%) | ||||||
| Mobiluncus (0.32%) | ||||||
| Azorhizophilus (0.18%) | ||||||
| Ralstonia (0.12%) | ||||||
| Hong et al. (2016) | 30 | Korea | Vaginal swab | 454 pyrosequencing (V3-V5) | Lactobacillus (83.41%) | [28] |
| Streptococcus (4.90%) | ||||||
| Diaphorobacter (2.50%) | ||||||
| Enterobacteriaceae (1.97%) | ||||||
| Cupriavidus (1.36%) | ||||||
| Prevotella (0.80%) | ||||||
| Cloacibacterium (0.43%) | ||||||
| Veillonella (0.34%) | ||||||
| Chlamydia (0.22%) | ||||||
| Comamonas (0.20%) | ||||||
| Novosphingobium (0.18%) | ||||||
| Staphylococcus (0.16%) | ||||||
| Haemophilus (0.14%) | ||||||
| Gemella (0.13%) | ||||||
| Pseudomonas (0.11%) | ||||||
| Acinetobacter (0.10%) | ||||||
| Moreno et al. (2016) | 26 | Spain | Vaginal aspirates | 454 pyrosequencing (V3-V5) | Lactobacillus | [18] |
| Gardnerella | ||||||
| Atopobium | ||||||
| Prevotella | ||||||
| Sneathia | ||||||
| Campisciano et al. (2017) | 30 | Italy | Cervico-vaginal fluid | Ion Torrent PGM (V1-V3) | Firmicutes; Bacilli (97%) | [29] |
| Proteobacteria; Gammaproteobacteria (1%) | ||||||
| Bacteria; Actinobacteria | ||||||
| Bacteria; Tenericutes | ||||||
| Bradley et al. (2018) | 47 | Sweden | Cervicovaginal swab | 454 pyrosequencing (V3-V4) | Lactobacillus (67.6%) | [30] |
| Gardnerella (17.4%) | ||||||
| Atopobium (5.6%) | ||||||
| Megasphaera (3.3%) | ||||||
| Prevotella (2.2%) | ||||||
| Sneathia | ||||||
| Coriobacteriaceae | ||||||
| Veillonella | ||||||
| Clostridium | ||||||
| Brotman et al. (2018) | 30 | United States | Vaginal swab | 454 pyrosequencing (V1-V2) | Lactobacillus (83%) | [31] |
| Chen et al. (2018) | 19 | Taiwan | Vaginal swab | Illumina MiSeq (V4) | Lactobacillus (74%) | [32] |
| Bifidobacterium (7%) | ||||||
| Gardnerella | ||||||
| Prevotella | ||||||
| Atopobium | ||||||
| Escherichia | ||||||
| Dialister | ||||||
| Kyono et al. (2018) | 15 | Japan | Vaginal discharge (swab) | Illumina MiSeq (V4) | Lactobacillus (99.80%) | [19] |
| Matsumoto et al. (2018) | 22 | Japan | Vaginal swab | Illumina MiSeq (V3-V4) | Lactobacillus | [33] |
| Bifidobacterium | ||||||
| Gardnerella | ||||||
| Bacteroides | ||||||
| Escherichia | ||||||
| Enterococcus | ||||||
| Clostridium | ||||||
| Ata et al. (2019) | 14 | Turkey | Vaginal swab | Illumina MiSeq (V3-V4) | Lactobacillus | [23] |
| Gardnerella | ||||||
| Prevotella | ||||||
| Gemella | ||||||
| Megasphaera | ||||||
| Atopobium | ||||||
| Ureaplasma | ||||||
| Dialister | ||||||
| Sneathia | ||||||
| Ceccarani et al. (2019) | 21 | Italy | Vaginal swab | Illumina MiSeq (V3-V4) | Lactobacillus (79.16%) | [34] |
| Gardnerella (2.72%) | ||||||
| Uncl. Clostridiales (1.66%) | ||||||
| Faecalibacterium (1.49%) | ||||||
| Ruminococcaceae (other) (1.35%) | ||||||
| Prevotella (1.16%) | ||||||
| Roseburia (1.09%) | ||||||
| Uncl. Ruminococcaceae (1.08%) | ||||||
| Bacteroides (0.69%) | ||||||
| Oscillospira (0.65%) | ||||||
| Coprococcus (0.61%) | ||||||
| Ruminococcus (0.54%) | ||||||
| Anaerococcus (0.48%) | ||||||
| Streptococcus (0.40%) | ||||||
| Uncl. Lachnospiraceae (0.40%) | ||||||
| Dialister (0.37%) | ||||||
| Blautia (0.35%) | ||||||
| Peptoniphilus (0.35%) | ||||||
| Akkermansia (0.30%) | ||||||
| Porphyromonas (0.25%) | ||||||
| Ureaplasma (0.25%) | ||||||
| Bifidobacterium (0.20%) | ||||||
| Parvimonas (0.20%) | ||||||
| Sneathia (0.18%) | ||||||
| Atopobium (0.17%) | ||||||
| Clostridium (0.16%) | ||||||
| Escherichia (0.13%) | ||||||
| Uncl. Coriobacteriaceae (0.10%) | ||||||
| Hong et al. (2019) | 37 | China | Vaginal swab | Illumina HiSeq (V3-V4) | Lactobacillus | [35] |
| Gardnerella | ||||||
| Atopobium | ||||||
| Prevotella | ||||||
| Streptococcus | ||||||
| Sneathia | ||||||
| Lin et al. (2019) | 16 | China | Vaginal secretion | Illumina MiSeq (V3-V4) | Lactobacillus (43.88%) | [36] |
| Bifidobacteriaceae (16.54%) | ||||||
| Streptococcus (9.82%) | ||||||
| Coriobacteriaceae (7.22%) | ||||||
| Liu et al. (2019) | 30 | China | Vaginal swab | Illumina HiSeq (V4) | Lactobacillus (>97%) | [37] |
| Xu et al. (2019) | 32 | China | Vaginal swab | Illumina MiSeq (V3-V4) | Lactobacillus (83.80%) | [38] |
| Gardnerella (3.19%) | ||||||
| Sneathia (2.26%) | ||||||
| Zhou et al. (2019) | 42 | China | Vaginal swab | Illumina MiSeq (V3-V4) | Lactobacillus (86.59%) | [39] |
| Gardnerella (3.26%) | ||||||
| Pseudomonas (3.23%) | ||||||
| Prevotella (2.01%) | ||||||
| Atopobium (1.70%) | ||||||
| Dialister (0.24%) | ||||||
| Anaerococcus (0.23%) | ||||||
| Aerococcus (0.18%) | ||||||
| Stenotrophomonas (0.17%) | ||||||
| Megasphaera (0.16%) | ||||||
| Bacteroides (0.13%) | ||||||
| Chen et al. (2020) | 68 | China | Vaginal swab | Illumina MiSeq (V3-V4) | Lactobacillus (64.93%) | [40] |
| Gardnerella | ||||||
| Prevotella (5.91%) | ||||||
| Atopobium (3.12%) | ||||||
| Sneathia (2.39%) | ||||||
| Anaerococcus (1.22%) | ||||||
| Streptococcus (1.03%) | ||||||
| Megasphaera (1.01%) | ||||||
| Bacillus (0.34%) | ||||||
| Chorna et al. (2020) | 8 | Puerto Rico | Vaginal swab | Not specified | Lactobacillus | [24] |
| Shuttleworthia | ||||||
| Gardnerella | ||||||
| Atopobium | ||||||
| Prevotella | ||||||
| Megasphaera | ||||||
| Sneathia | ||||||
| Tu et al. (2020) | 50 | China | Vaginal swab | Illumina MiSeq (V3-V4) | Lactobacillus | [25] |
| Gardnerella | ||||||
| Atopobium | ||||||
| Wang et al. (2020) | 160 | China | Vaginal swab | Illumina HiSeq (V4) | Lactobacillus (95.90%) | [41] |
| Gardnerella | ||||||
| Pseudomonas | ||||||
| Streptococcus | ||||||
| Aerococcus | ||||||
| Atopobium | ||||||
| Prevotella | ||||||
| Wang et al. (2020) | 29 | China | Vaginal swab | Illumina MiSeq (V3-V4) | Lactobacillus | [42] |
| Gardnerella | ||||||
| Bacteroides | ||||||
| Prevotella | ||||||
| Atopobium | ||||||
| Xie et al. (2020) | 27 | China | Vaginal swab | Illumina MiSeq (V4) | Lactobacillus | [43] |
| Acinetobacter | ||||||
| Megasphaera | ||||||
| Pseudomonas | ||||||
| Ochrobactrum | ||||||
| Sneathia | ||||||
| Zhao et al. (2020) | 92 | China | Vaginal swab | Illumina HiSeq (V1-V2) | Lactobacillus | [44] |
| Bifidobacterium | ||||||
| Prevotella | ||||||
| Atopobium | ||||||
| Bacteroides | ||||||
| Streptococcus | ||||||
| Clostridium | ||||||
| Sirichoat et al. (2021) | 51 | Thailand | Vaginal swab | Ion Torrent PGM (V2, V3, V4, V6-7, V8, V9) | Lactobacillus (78%) | [45] |
| Gardnerella (14%) | ||||||
| Atopobium (2%) | ||||||
| Pseudomonas (2%) | ||||||
Summary of the diversity of the female genital tract microbiome in healthy women.
Diversity of the Female Genital Tract Microbiome in Women With Health Conditions Associated With Ectopic Pregnancy
Studies included for women with health conditions were cross-sectional except for an observational prospective study investigating the vaginal microbiome in women with failed intrauterine insemination [46]. Meanwhile, the sample size of the studies also varied with a range of 1–118. Not all studies provided numerical values of relative abundance and these were ranked according to descending abundance. For standardisation, the lowest taxonomic rank observed in our review is the genus level while taxa with a relative abundance of less than 0.1% were not tabulated. Table 2 summarises the relative abundance of vaginal microbiome in various reproductive conditions while Table 3 outlines the changes in relative abundance in comparison with healthy groups. Table 4 summarises the cervical microbiome’s relative abundance in reproductive conditions while Table 5 compares the relative abundance with healthy groups. Table 6 highlights the relative abundance of uterine microbiome in reproductive conditions while Table 7 shows the comparison of uterine microbiome relative abundance in disease state with healthy controls. Overall, there was a decrease in the relative abundance of the genus Lactobacillus in the disease state and an increase in various other genera in the vaginal and cervical microbiome (Figure 1). Meanwhile looking at the uterine microbiome, various sampling methods were used, with inconsistent findings across studies. However, in general, there was a decrease in the phylum Proteobacteria and an increase in the other taxa (Figure 2).
TABLE 2
| Reproductive condition | Author (Year) | Genital microbiome relative abundance (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | Bacteroidetes | Candidatus Saccharibacteria | Cyanobacteria | Firmicutes | Fusobacteria | Proteobacteria | Tenericutes | Verrucomicrobia | ||
| Tubal pregnancy | Ruan (2021) [47] | Gardnerella 12 | Prevotella 6 | Lactobacillus 62 | Sneathia 3 | |||||
| Atopobium 4 | Megasphaera 2 | |||||||||
| Chronic endometritis | Lozano (2021) [48] | Prevotella 0.98 | Lactobacillus 87.44 | Escherichia 0.17 | ||||||
| Streptococcus 9.44 | ||||||||||
| Dialister 0.68 | ||||||||||
| Veillonella 0.68 | ||||||||||
| Chlamydia trachomatis | Ceccarani (2019) [34] | Gardnerella 3.65 | Prevotella 1.6 | Lactobacillus 67.45 | Sneathia 0.41 | Escherichia 0.33 | Akkermansia 0.39 | |||
| Atopobium 1 | Bacteroides 0.91 | Roseburia 4.42 | Haemophilus 0.1 | |||||||
| Bifidobacterium 0.46 | Megasphaera 2.97 | |||||||||
| Coriobacteriaceae, unclassifieda 0.19 | Faecalibacterium 2.31 | |||||||||
| Ruminococcaceaea 1.95 | ||||||||||
| Clostridiales, unclassifieda 1.56 | ||||||||||
| Ruminococcaceae, unclassifieda 1.03 | ||||||||||
| Blautia 0.91 | ||||||||||
| Coprococcus 0.66 | ||||||||||
| Clostridium 0.65 | ||||||||||
| Lachnospiraceae, unclassifieda 0.59 | ||||||||||
| Ruminococcus 0.58 | ||||||||||
| Dialister 0.56 | ||||||||||
| Oscillospira 0.56 | ||||||||||
| Shuttleworthia 0.54 | ||||||||||
| Streptococcus 0.49 | ||||||||||
| Aerococcus 0.24 | ||||||||||
| Peptoniphilus 0.15 | ||||||||||
| Vulvovaginal candidiasis | Ceccarani (2019) [34] | Gardnerella 7.68 | Prevotella 3.76 | Lactobacillus 56.69 | Sneathia 0.53 | Haemophilus 1.42 | Ureaplasma 0.41 | Akkermansia 0.35 | ||
| Atopobium 1.94 | Bacteroides 0.81 | Roseburia 3.51 | Escherichia 0.4 | |||||||
| Bifidobacterium 1.28 | Faecalibacterium 2.14 | |||||||||
| Alloscardovia 0.57 | Ruminococcaceaea 1.86 | |||||||||
| Coriobacteriaceae, | Aerococcus 1.5 | |||||||||
| unclassifieda0.24 | Clostridiales, unclassifieda 1.44 | |||||||||
| Megasphaera 1.04 | ||||||||||
| Streptococcus 1.04 | ||||||||||
| Ruminococcaceae, unclassifieda 1.02 | ||||||||||
| Dialister 0.78 | ||||||||||
| Blautia 0.77 | ||||||||||
| Coprococcus 0.63 | ||||||||||
| Ruminococcus 0.59 | ||||||||||
| Lachnospiraceae, unclassifieda 0.54 | ||||||||||
| Oscillospira 0.53 | ||||||||||
| Gemellaceae, unclassifieda 0.49 | ||||||||||
| Veillonella 0.46 | ||||||||||
| Anaerococcus 0.46 | ||||||||||
| Finegoldia 0.45 | ||||||||||
| Gemella 0.37 | ||||||||||
| Clostridium 0.32 | ||||||||||
| Shuttleworthia 0.31 | ||||||||||
| Parvimonas 0.17 | ||||||||||
| Peptoniphilus 0.13 | ||||||||||
| LR-HPV Infection | Zhou (2019) [39] | Gardnerella 10.83 | Prevotella 4.17 | Lactobacillus 49.95 | Sneathia 5.69 | Pseudomonas 1.57 | ||||
| Atopobium 4.62 | Bacteroides 1.89 | Saccharofermentans 1.33 | Fusobacterium 0.66 | Hydrogenophilus 0.55 | ||||||
| Bifidobacterium 2.43 | Megasphaera 1.12 | Burkholderia 0.48 | ||||||||
| Corynebacterium 1.33 | Peptostreptococcus 0.62 | Escherichia/Shigella 0.30 | ||||||||
| Stenotrophomonas 0.57 | ||||||||||
| Dialister 0.42 | ||||||||||
| Aerococcus 0.27 | ||||||||||
| Anaerococcus 0.25 | ||||||||||
| Bacterial vaginosis | Ceccarani (2019) [34] | Gardnerella 11.44 | Prevotella 9.15 | Rs-045, unclassifieda 0.43 | Lactobacillus 18.8 | Sneathia 7.76 | Escherichia 0.23 | Akkermansia 0.32 | ||
| Atopobium 4.92 | Bacteroides 0.86 | Megasphaera 8.64 | ||||||||
| Coriobacteriaceae, unclassifieda 0.89 | Porphyromonas 0.73 | Shuttleworthia 7.48 | ||||||||
| Mobiluncus 0.49 | Roseburia 3.51 | |||||||||
| Bifidobacterium 0.33 | Clostridium 2.14 | |||||||||
| Faecalibacterium 2.09 | ||||||||||
| Aerococcus 2.06 | ||||||||||
| Dialister 2.02 | ||||||||||
| Ruminococcaceaea 1.81 | ||||||||||
| Clostridiales, unclassifieda 1.58 | ||||||||||
| Parvimonas 1.39 | ||||||||||
| Peptoniphilus 1.09 | ||||||||||
| Ruminococcaceae, unclassifieda 1.03 | ||||||||||
| Blautia 0.74 | ||||||||||
| Peptostreptococcus 0.63 | ||||||||||
| Coprococcus 0.59 | ||||||||||
| Oscillospira 0.58 | ||||||||||
| Ruminococcus 0.56 | ||||||||||
| Streptococcus 0.54 | ||||||||||
| Lachnospiraceae, unclassifieda 0.52 | ||||||||||
| Anaerococcus 0.39 | ||||||||||
| Gemella 0.27 | ||||||||||
| Finegoldia 0.16 | ||||||||||
| Bacterial vaginosis | Hong (2016) [28] | Atopobium 4.46 | Prevotella 27.80 | Lactobacillus 38.98 | Sneathia 7.48 | Diaphorobacter 1.67 | Mycoplasma 0.35 | |||
| Gardnerella 1.36 | Porphyromonas 1.29 | Aerococcus 5.62 | Fusobacterium 0.30 | Cupriavidus 1.03 | ||||||
| Mobiluncus 0.69 | Megasphaera 1.72 | |||||||||
| Coriobacteriaceae, unclassifieda 0.38 | Dialister 1.05 | |||||||||
| Saccharofermentans 0.94 | ||||||||||
| Peptoniphilus 0.69 | ||||||||||
| Anaerococcus 0.55 | ||||||||||
| Moryella 0.35 | ||||||||||
| Vaginosis | Campisciano (2017) [29] | Actinobacteriaa 16 | Bacteroidiaa 5 | Bacillia 71 | Fusobacteriaa 1 | Gammaproteobacteriaa 4 | Tenericutesa 1 | |||
| Clostridiaa 1 | ||||||||||
| Aerobic vaginitis | Wang (2019) [41] | Gardnerella | Prevotella | Lactobacillus 41.6 | Sneathia | Klebsiella 0.5 | Ureaplasma 0.3 | |||
| Atopobium | Streptococcus | Escherichia | Mycoplasma 0.12 | |||||||
| Bifidobacterium | Aerococcus | |||||||||
| Alloscardovia | Anaerococcus | |||||||||
| Eubacterium | ||||||||||
| Veillonella | ||||||||||
| Megasphaera | ||||||||||
| Dialister | ||||||||||
| Empty-sac miscarriage | Liu (2021) [49] | Bacteroides | Lactobacillus | Halomonas | ||||||
| Missed miscarriage | Liu (2021) [49] | Bacteroides | Cyanobacteriaa | Lactobacillus | Fusobacterium | Halomonas | ||||
| Lachnospiraceaea | Escherichia/Shigella | |||||||||
| Bacillus | Succinivibrio | |||||||||
| Staphylococcus | Burkhoderia | |||||||||
| Acetobacter | ||||||||||
| Embryonic miscarriage | Xu (2020) [50] | Bifidobacterium | Bacteroides | Lactobacillus | Escherichia-Shigella | |||||
| Gardnerella | Parabacteroides | Faecalibacterium | ||||||||
| Alistipes | Lachnospiraceaea | |||||||||
| Roseburia | ||||||||||
| ART failure | Bernabeu (2019) [51] | Gardnerella | Lactobacillus | Ureaplasma | ||||||
| Streptococcus | ||||||||||
| Clostridium | ||||||||||
| IUI failure | Amato (2020) [46] | Bifidobacteriaceaea 12 | Lactobacillaceaea 83 | |||||||
| IVF failure | Kong (2020) [52] | Gardnerella 7.24 | Prevotella 3.02 | Lactobacillus 63.09 | Sneathia 3.75 | Proteobacteriaa 8.01 | ||||
| Atopobium 4.14 | Streptococcus | |||||||||
| Megasphaera | ||||||||||
| Aerococcus | ||||||||||
| Infertility | Riganelli (2020) [53] | Bifidobacterium | Prevotella | Lactobacillus | Escherichia | |||||
| Gardnerella | Streptococcus | |||||||||
| Atopobium | Shuttleworthia | |||||||||
| Zhao (2020) [44] | Bifidobacterium | Prevotella | Lactobacillus | |||||||
| Atopobium | Aerococcus | |||||||||
| Idiopathic infertility | Campisciano (2017) [29] | Actinobacteriaa 8 | Bacteroidiaa 1 | Bacillia 84 | Gammaproteobacteriaa 3 | Tenericutesa 2 | ||||
| Clostridiaa 1 | ||||||||||
| Diagnosed infertility | Campisciano (2017) [29] | Actinobacteriaa 5 | Bacillia 71 | Gammaproteobacteriaa 23 | ||||||
| Clostridiaa 1 | ||||||||||
| Deep endometriosis | Hernandes (2020) [54] | Gardnerella | Prevotella | Lactobacillus | Pseudomonas | Ureaplasma | ||||
| Corynebacterium | Streptococcus | Alishewanella | ||||||||
| Enterococcus | ||||||||||
| Anaerococcus | ||||||||||
| PCOS | Hong (2021) [55] | Gardnerella 10.4 | Prevotella 7.94 | Lactobacillus 58.52 | Sneathia 1.57 | Mycoplasma 1.25 | ||||
| Atopobium 4.36 | Streptococcus 2.76 | |||||||||
| Bifidobacterium 1.55 | Megasphaera 1.54 | |||||||||
| Tu (2020) [25] | Gardnerella | Prevotella | Lactobacillus | Sneathia | Escherichia-Shigella | Ureaplasma | ||||
| Atopobium | Porphyromonas | Streptococcus | Fusobacterium | Campylobacter | Mycoplasma | |||||
| Bifidobacterium | Aerococcus | Acinetobacter | ||||||||
| Corynebacterium | Dialister | |||||||||
| Lawsonella | Peptoniphilus | |||||||||
| Finegoldia | ||||||||||
| Anaerococcus | ||||||||||
| Veillonella | ||||||||||
| Megasphaera | ||||||||||
| Peptostreptococcus | ||||||||||
| Varibaculum | ||||||||||
| Staphylococcus | ||||||||||
| Ezakiella | ||||||||||
| Intrauterine adhesion | Liu (2019) [37] | Actinobacteriaa 24.37 | Bacteroidetesa 8.64 | Firmicutesa 61.84 | Proteobacteriaa 2.74 | |||||
Relative abundance of the vaginal microbiome in various reproductive conditions.
Unknown genera.
TABLE 3
| Reproductive condition | Author (Year) | Genital microbiome relative abundance (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | Bacteroidetes | Candidatus Saccharibacteria | Firmicutes | Fusobacteria | Proteobacteria | Tenericutes | Verrucomicrobia | ||
| Tubal pregnancy | Ruan (2021) [47] | Gardnerella ↑ | Prevotella ↑ | Lactobacillus | Sneathia | ||||
| Atopobium | Megasphaera | Leptotrichiaceaea ↑ | |||||||
| Clostridiaa ↑ | |||||||||
| Chronic endometritis | Lozano (2021) [48] | Prevotella | Lactobacillus ↓ | Escherichia | |||||
| Streptococcus ↑ | |||||||||
| Dialister | |||||||||
| Veillonella | |||||||||
| Chlamydia trachomatis | Ceccarani (2019) [34] | Gardnerella | Prevotella | Lactobacillus | Sneathia ↑ | Escherichia ↑ | Akkermansia | ||
| Atopobium ↑ | Bacteroides ↑ | Roseburia ↑ | Haemophilus | ||||||
| Bifidobacterium ↑ | Megasphaera ↑ | ||||||||
| Coriobacteriaceae, unclassifieda ↑ | Faecalibacterium ↑ | ||||||||
| Ruminococcaceaea ↑ | |||||||||
| Clostridiales, unclassifieda | |||||||||
| Ruminococcaceae, unclassifieda | |||||||||
| Blautia ↑ | |||||||||
| Coprococcus ↑ | |||||||||
| Clostridium ↑ | |||||||||
| Lachnospiraceae, unclassifieda ↑ | |||||||||
| Ruminococcus | |||||||||
| Dialister ↑ | |||||||||
| Oscillospira | |||||||||
| Shuttleworthia ↑ | |||||||||
| Streptococcus ↑ | |||||||||
| Aerococcus ↑ | |||||||||
| Peptoniphilus | |||||||||
| Vulvovaginal candidiasis | Ceccarani (2019) [34] | Gardnerella ↑ | Prevotella ↑ | Lactobacillus ↓ | Sneathia | Haemophilus | Ureaplasma | Akkermansia | |
| Atopobium ↑ | Bacteroides ↑ | Roseburia | Escherichia ↑ | ||||||
| Bifidobacterium ↑ | Faecalibacterium ↑ | ||||||||
| Alloscardovia ↑ | Ruminococcaceaea ↑ | ||||||||
| Coriobacteriaceae, unclassifieda ↑ | Aerococcus | ||||||||
| Clostridiales, unclassifieda | |||||||||
| Megasphaera ↑ | |||||||||
| Streptococcus ↑ | |||||||||
| Ruminococcaceae, unclassifieda | |||||||||
| Dialister ↑ | |||||||||
| Blautia ↑ | |||||||||
| Coprococcus ↑ | |||||||||
| Ruminococcus | |||||||||
| Lachnospiraceae, unclassifieda ↑ | |||||||||
| Oscillospira | |||||||||
| Gemellaceae, unclassifieda | |||||||||
| Veillonella ↑ | |||||||||
| Anaerococcus | |||||||||
| Finegoldia ↑ | |||||||||
| Gemella | |||||||||
| Clostridium | |||||||||
| Shuttleworthia ↑ | |||||||||
| Parvimonas | |||||||||
| Peptoniphilus | |||||||||
| LR-HPV Infection | Zhou (2019) [39] | Gardnerella ↑ | Prevotella | Lactobacillus ↓ | Sneathia ↑ | Pseudomonas | |||
| Atopobium | Bacteroides | Saccharofermentans | Fusobacterium | Hydrogenophilus | |||||
| Bifidobacterium | Megasphaera | Burkholderia | |||||||
| Corynebacterium | Peptostreptococcus | Escherichia/Shigella | |||||||
| Stenotrophomonas | |||||||||
| Dialister | |||||||||
| Aerococcus | |||||||||
| Anaerococcus | |||||||||
| Bacterial vaginosis | Ceccarani (2019) [34] | Gardnerella ↑ | Prevotella ↑ | Rs-045, unclassifieda | Lactobacillus ↓ | Sneathia ↑ | Escherichia | Akkermansia | |
| Atopobium ↑ | Bacteroides ↑ | Megasphaera ↑ | |||||||
| Coriobacteriaceae, unclassifieda ↑ | Porphyromonas | Shuttleworthia ↑ | |||||||
| Mobiluncus ↑ | Roseburia | ||||||||
| Bifidobacterium | Clostridium | ||||||||
| Faecalibacterium | |||||||||
| Aerococcus ↑ | |||||||||
| Dialister ↑ | |||||||||
| Ruminococcaceaea | |||||||||
| Clostridiales, unclassifieda | |||||||||
| Parvimonas | |||||||||
| Peptoniphilus | |||||||||
| Ruminococcaceae, unclassifieda | |||||||||
| Blautia | |||||||||
| Peptostreptococcus ↑ | |||||||||
| Coprococcus | |||||||||
| Oscillospira | |||||||||
| Ruminococcus | |||||||||
| Streptococcus | |||||||||
| Lachnospiraceae, unclassifieda ↑ | |||||||||
| Anaerococcus | |||||||||
| Gemella ↑ | |||||||||
| Finegoldia | |||||||||
| Vaginosis | Campisciano (2017) [29] | Actinobacteriaa | Bacteroidiaa | Bacillia ↓ | Fusobacteriaa | Gammaproteobacteriaa ↑ | Tenericutesa ↑ | ||
| Clostridiaa | |||||||||
| Aerobic vaginitis | Wang (2019) [41] | Gardnerella ↑ | Prevotella ↑ | Lactobacillus ↓ | Sneathia | Klebsiella | Ureaplasma | ||
| Atopobium ↑ | Bacteroidetesa | Streptococcus ↑ | Escherichia | Mycoplasma | |||||
| Bifidobacterium | Aerococcus ↑ | ||||||||
| Alloscardovia | Anaerococcus | ||||||||
| Actinobacteriaa | Eubacterium | ||||||||
| Veillonella | |||||||||
| Megasphaera | |||||||||
| Dialister | |||||||||
| Embryonic miscarriage | Xu (2020) [50] | Bifidobacterium | Bacteroides | Lactobacillus | Fusobacteriaa ↓ | Escherichia-Shigella | |||
| Gardnerella | Parabacteroides | Faecalibacterium | |||||||
| Alistipes | Lachnospiraceaea ↑ | ||||||||
| Roseburia ↑ | |||||||||
| IUI failure | Amato (2020) [46] | Bifidobacteriaceaea ↑ | Lactobacillaceaea ↓ | ||||||
| Idiopathic infertility | Campisciano (2017) [29] | Actinobacteriaa ↑ | Bacteroidiaa | Bacillia | Gammaproteobacteriaa | Tenericutesa | |||
| Clostridiaa | |||||||||
| PCOS | Tu (2020) [25] | Gardnerella | Prevotella ↑ | Lactobacillus | Sneathia | Escherichia-Shigella | Ureaplasma | ||
| Atopobium | Porphyromonas ↑ | Streptococcus | Fusobacterium | Campylobacter | Mycoplasma ↑ | ||||
| Bifidobacterium | Aerococcus | Acinetobacter | |||||||
| Corynebacterium | Dialister | ||||||||
| Lawsonella | Peptoniphilus ↑ | ||||||||
| Finegoldia | |||||||||
| Anaerococcus | |||||||||
| Veillonella | |||||||||
| Megasphaera | |||||||||
| Peptostreptococcus | |||||||||
| Varibaculum | |||||||||
| Staphylococcus | |||||||||
| Ezakiella | |||||||||
| Intrauterine adhesion | Liu (2019) [37] | Actinobacteriaa | Bacteroidetesa ↑ | Firmicutesa | Proteobacteriaa | ||||
Comparison of the vaginal microbiome relative abundance in disease state with healthy controls.
Unknown genera.
TABLE 4
| Reproductive condition | Author (Year) | Genital microbiome relative abundance (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | Bacteroidetes | Chlamydiae | Cyanobacteria | Firmicutes | Fusobacteria | Proteobacteria | Tenericutes | ||
| Chlamydia trachomatis | Di Pietro (2018) [21] | Gardnerella 14 | Prevotella 6 | Lactobacillus 50 | Leptotrichia 21 | ||||
| Filardo (2019) [56] | Gardnerella 15.5 | Prevotella 6.5 | Lactobacillus 51.1 | ||||||
| Aerococcus 1 | |||||||||
| Asymptomatic Chlamydia trachomatis | Filardo (2017) [20] | Gardnerella 14.3 | Prevotella 0.5 | Chlamydia | Lactobacillus 60 | Leptotrichia 10 | Escherichia | Ureaplasma | |
| Atopobium | Megasphera | Fusobacterium | Mycoplasma | ||||||
| 10 Bifidobacterium | Dialister | ||||||||
| Streptococcus | |||||||||
| Aerococcus | |||||||||
| Parvimonas | |||||||||
| HPV/CT | Di Pietro (2018) [21] | Gardnerella 19 | Bacteroidetesa<1 | Firmicutesa 63 | Fusobacteriaa <1 | ||||
| Atopobium 4 | |||||||||
| HPV | Di Pietro (2018) [21] | Actinobacteriaa 1.3 | Bacteroidetesa<1 | Firmicutesa 98 | Fusobacteriaa <1 | Proteobacteriaa <1 | Tenericutesa<1 | ||
| HPV infection - LSIL | Kwasniewski (2018) [57] | Actinobacteriaa 1 | Bacillia 84 | Gammproteobacteriaa 8.2 | |||||
| Clostridiaa 0.1 | |||||||||
| HPV infection—HSIL | Kwasniewski (2018) [57] | Actinobacteriaa 8.1 | Nostocophycideaea 0.15 | Bacillia 27.69 | Gammproteobacteriaa 61.48 | ||||
| Clostridiaa 0.2 | Alphaproteobacteriaa 0.41 | ||||||||
| Infectious infertility | Graspeuntner (2018) [22] | Gardnerella 10.08 | Prevotella 7.37 | Lactobacillus 57.74 | Sneathia 2.58 | Enterobacteriaceae, unclassifieda 0.27 | Mycoplasma 1.71 | ||
| Atopobium 2.18 | Porphyromonas 0.27 | Streptococcus 5.5 | |||||||
| Corynebacterium 1.28 | Lachnospiraceaea 1.69 | ||||||||
| Bifidobacterium 0.18 | Veillonella 1.63 | ||||||||
| Dialister 1.25 | |||||||||
| Aerococcus 0.48 | |||||||||
| Non-infectious infertility | Graspeuntner (2018) [22] | Gardnerella 5.61 | Prevotella 3.93 | Lactobacillus 69.01 | Sneathia 0.5 | Enterobacteriaceae, unclassifieda 0.98 | Mycoplasma 0.02 | ||
| Atopobium 2.72 | Lachnospiraceaea 6.59 | ||||||||
| Bifidobacterium 0.21 | Aerococcus 1.49 | ||||||||
| Dialister 0.94 | |||||||||
| Veillonella 0.76 | |||||||||
| Streptococcus 0.65 | |||||||||
| Clostridiales, unclassifieda 0.37 | |||||||||
| Stage 3 endometriosis | Cregger (2017) [11] | Barnesiella 19.75 | Clostridium XIVa 2.25 | Sneathia 0.25 | Achromobacter 0.75 | ||||
| Bacteroides 1.75 | Staphylococcus 1.5 | ||||||||
| Tannerella 1.75 | Coprococcus 1.25 | ||||||||
| Parabacteroides 1.25 | Propionibacterium 1.25 | ||||||||
| Alkalitalea 0.75 | Allobaculum 1 | ||||||||
| Butyricicoccus 1 | |||||||||
| Acetivibrio 0.75 | |||||||||
| Anaerotruncus 0.75 | |||||||||
| Ruminococcus 0.75 | |||||||||
| Turicibacter 0.75 | |||||||||
| Coprobacillus 0.5 | |||||||||
| Lactobacillus 0.5 | |||||||||
| Clostridium XIVb 0.25 | |||||||||
| Flavonifractor 0.25 | |||||||||
| Endometriosis—other stages | Cregger (2017) [11] | Barnesiella 0.93 | |||||||
| Stage 3–4 endometriosis | Ata (2019) [23] | 2 Gardnerella | 3 Prevotella | Lactobacillus | |||||
| 5 Atopobium | Dialister | ||||||||
| Streptococcus | |||||||||
| PCOS | Tu (2020) [25] | Gardnerella | Prevotella | Lactobacillus | Sneathia | Escherichia-Shigella | Ureaplasma | ||
| Atopobium | Porphyromonas | Streptococcus | Fusobacterium | Campylobacter | Mycoplasma | ||||
| Bifidobacteriu | Finegoldia | Acinetobacter | |||||||
| Varibaculum | Peptoniphilus | Sutterella | |||||||
| Corynebacterium | Aerococcus | ||||||||
| Dialister | |||||||||
| Megasphaera | |||||||||
| Anaerococcus | |||||||||
| Veillonella | |||||||||
| Peptostreptococcus | |||||||||
| Staphylococcus | |||||||||
Relative abundance of the cervical microbiome in various reproductive conditions.
Unknown genera.
TABLE 5
| Reproductive condition | Author (Year) | Genital microbiome relative abundance (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Actinobacteria | Bacteroidetes | Chlamydiae | Firmicutes | Fusobacteria | Proteobacteria | Tenericutes | ||
| Chlamydia trachomatis | Di Pietro (2018) [21] | Gardnerella ↑ | Prevotella ↑ | Lactobacillus ↓ | Leptotrichia | |||
| Filardo (2019) [56] | Gardnerella ↑ | Prevotella ↑ | Lactobacillus ↓ | |||||
| Aerococcus | ||||||||
| Asymptomatic Chlamydia trachomatis | Filardo (2017) [20] | Gardnerella ↑ | Prevotella 0.5 ↑ | Chlamydia | Lactobacillus ↓ | Leptotrichia ↑ | Escherichia | Ureaplasma |
| Atopobium ↑ | Megasphera | Fusobacterium | Mycoplasma | |||||
| Bifidobacterium | Dialister | |||||||
| Streptococcus | ||||||||
| Aerococcus | ||||||||
| Parvimonas | ||||||||
| HPV/CT | Di Pietro (2018) [21] | Gardnerella ↑ | Bacteroidetesa | Firmicutesa | Fusobacteriaa | |||
| Atopobium ↑ | ||||||||
| PCOS | Tu (2020) [25] | Gardnerella ↑ | Prevotella ↑ | Lactobacillus ↓ | Sneathia | Escherichia-Shigella | Ureaplasma | |
| Atopobium | Porphyromonas ↑ | Streptococcus | Fusobacterium | Campylobacter | Mycoplasma | |||
| Bifidobacterium | Finegoldia ↑ | Acinetobacter | ||||||
| Varibaculum | Peptoniphilus ↑ | Sutterella | ||||||
| Corynebacterium | Aerococcus ↑ | |||||||
| Dialister ↑ | ||||||||
| Megasphaera | ||||||||
| Anaerococcus ↑ | ||||||||
| Veillonella | ||||||||
| Peptostreptococcus | ||||||||
| Staphylococcus | ||||||||
Comparison of the cervical microbiome relative abundance in disease state with healthy controls.
Unknown genera.
TABLE 6
| Type of sample | Reproductive condition | Author (Year) | Genital microbiome abundance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | Bacteroidetes | Cyanobacteria | Firmicutes | Proteobacteria | Tenericutes | Verrucomicrobia | |||
| Endometrial fluid | Infertility | Vladislavovna (2020) [58] | Gardnerella 2.51 | Lactobacillus 34.37 | Ralstonia 7.23 | ||||
| Streptococcus 2.7 | Methylobacterium 2.92 | ||||||||
| Comamonas 2.87 | |||||||||
| Infertility (pipelle catheter) | Riganelli (2020) [53] | Actinobacteriaa | Bacteroidetea | Cyanobacteriaa | Firmicutesa | Proteobacteriaa | Verrucomicrobiaa | ||
| Infertility without chronic endometritis | Liu (2019) [59] | Gardnerella ∼8 | Prevotella ∼1 | Lactobacillus ∼58 | Stenotrophomonas ∼3 | ||||
| Atopobium ∼5 | Streptococcus ∼3 | Escherichia-Shigella ∼1 | |||||||
| Bifidobacterium ∼3 | Staphylococcus ∼1 | ||||||||
| Failure of implantation | Moreno (2016) [18] | Gardnerella | Lactobacillus | ||||||
| Bifidobacterium | Streptococcus | ||||||||
| Veillonella | |||||||||
| Clostridiales, unclassifieda | |||||||||
| Miscarriage in infertile women | Moreno (2016) [18] | Gardnerella | Lactobacillus | ||||||
| Bifidobacterium | Faecalibacterium | ||||||||
| Ruminococcus | |||||||||
| Roseburia | |||||||||
| Lachnospiraceaea | |||||||||
| Blautia | |||||||||
| Endometrial swab | Endometrial polyps | Fang (2016) [17] | Gardnerella 5.5 | Prevotella 1.3 | Lactobacillus 38.64 | Enterobacter 8.34 | |||
| Bifidobacterium 4.8 | Desulfosporosinus 4.23 | Pseudomonas 7.02 | |||||||
| Streptococcus 2.6 | Alteromonas 1.1 | ||||||||
| Enterobacteriaceae, unclassifieda 0.9 | |||||||||
| Sphingomonas 0.4 | |||||||||
| Endometrial polyps/Chronic endometritis | Fang (2016) [17] | Gardnerella 6.91 | Prevotella 1.3 | Lactobacillus 33.21 | Pseudomonas 7.32 | ||||
| Bifidobacterium 1.4 | Desulfosporosinus 5.41 | Enterobacter 7.17 | |||||||
| Streptococcus 1.1 | Alteromonas 1.4 | ||||||||
| Enterobacteriaceae, unclassifieda 1 | |||||||||
| Sphingomonas 0.6 | |||||||||
| Chronic endometritis | Lozano (2021) [48] | Gardnerella 4.05 | Lactobacillus 81.76 | Burkholderia 3.38 | |||||
| Anaerobacillus 2.03 | Ralstonia 2.7 | ||||||||
| Dialister 2.03 | Delftia 1.35 | ||||||||
| Streptococcus 2.03 | |||||||||
| Endometriosis | Khan (2016) [60] | Lactobacillaceaea 27 | Moraxellaceaea 15 | ||||||
| Streptococcaceaea 11 | Enterobacteriaceaea 1 | ||||||||
| Staphylococcaceaea 5 | |||||||||
| Endometrial tissue | Deep endometriosis | Hernandes (2020) [54] | Corynebacterium | Prevotella | Lactobacillus | Alishewanella | Ureaplasma | ||
| Gardnerella | Enterococcus | Pseudomonas | |||||||
| Anaerococcus | |||||||||
Relative abundance of the uterine microbiome relative abundance in various reproductive conditions.
Unknown genera.
TABLE 7
| Type of sample | Reproductive condition | Author (Year) | Genital microbiome relative abundance (%) | |||
|---|---|---|---|---|---|---|
| Actinobacteria | Bacteroidetes | Firmicutes | Proteobacteria | |||
| Endometrial fluid | Miscarriage in infertile women | Moreno (2016) [18] | Gardnerella ↑ | Lactobacillus | ||
| Bifidobacterium | Faecalibacterium | |||||
| Ruminococcus | ||||||
| Roseburia | ||||||
| Lachnospiraceaea | ||||||
| Blautia | ||||||
| Endometrial swab | Endometrial polyps | Fang (2016) [17] | Gardnerella | Prevotella | Lactobacillus | Enterobacter |
| Bifidobacterium | Desulfosporosinus | Pseudomonas | ||||
| Streptococcus | Alteromonas | |||||
| Firmicutesa ↑ | Enterobacteriaceae, unclassifieda | |||||
| Sphingomonas | ||||||
| Proteobacteriaa ↓ | ||||||
| Endometrial polyps/Chronic endometritis | Fang (2016) [17] | Gardnerella | Prevotella ↑ | Lactobacillus | Pseudomonas | |
| Bifidobacterium | Desulfosporosinus | Enterobacter ↓ | ||||
| Streptococcus | Alteromonas | |||||
| Firmicutesa ↑ | Enterobacteriaceae, unclassifieda | |||||
| Sphingomonas ↓ | ||||||
| Proteobacteriaa ↓ | ||||||
| Chronic endometritis | Lozano (2021) [48] | Gardnerella | Lactobacillus ↓ | Burkholderia | ||
| Anaerobacillus | Ralstonia | |||||
| Dialister ↓ | Delftia | |||||
| Streptococcus | ||||||
Comparison of the uterine microbiome relative abundance in disease state with healthy controls.
Unknown genera.
FIGURE 1
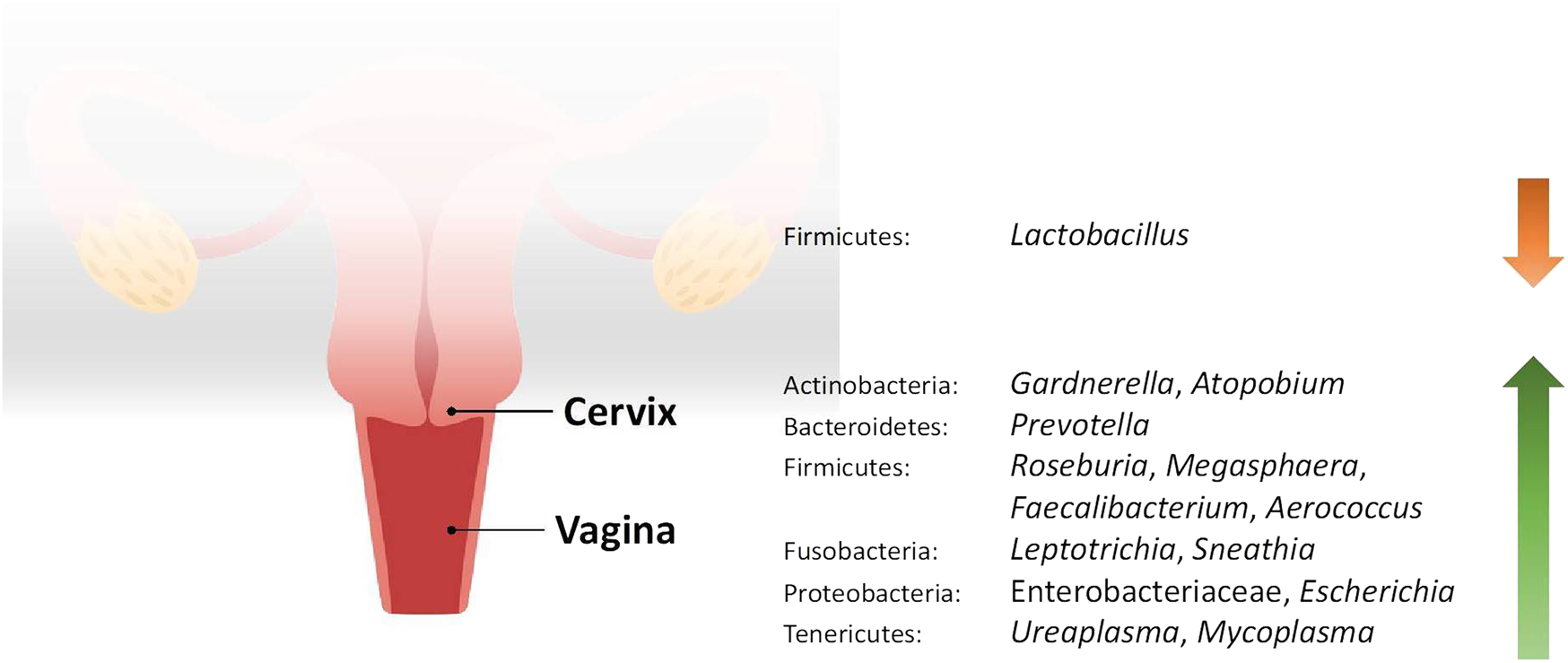
Changes in the relative abundance of the vaginal and cervical microbiome in reproductive conditions compared to the healthy controls.
FIGURE 2
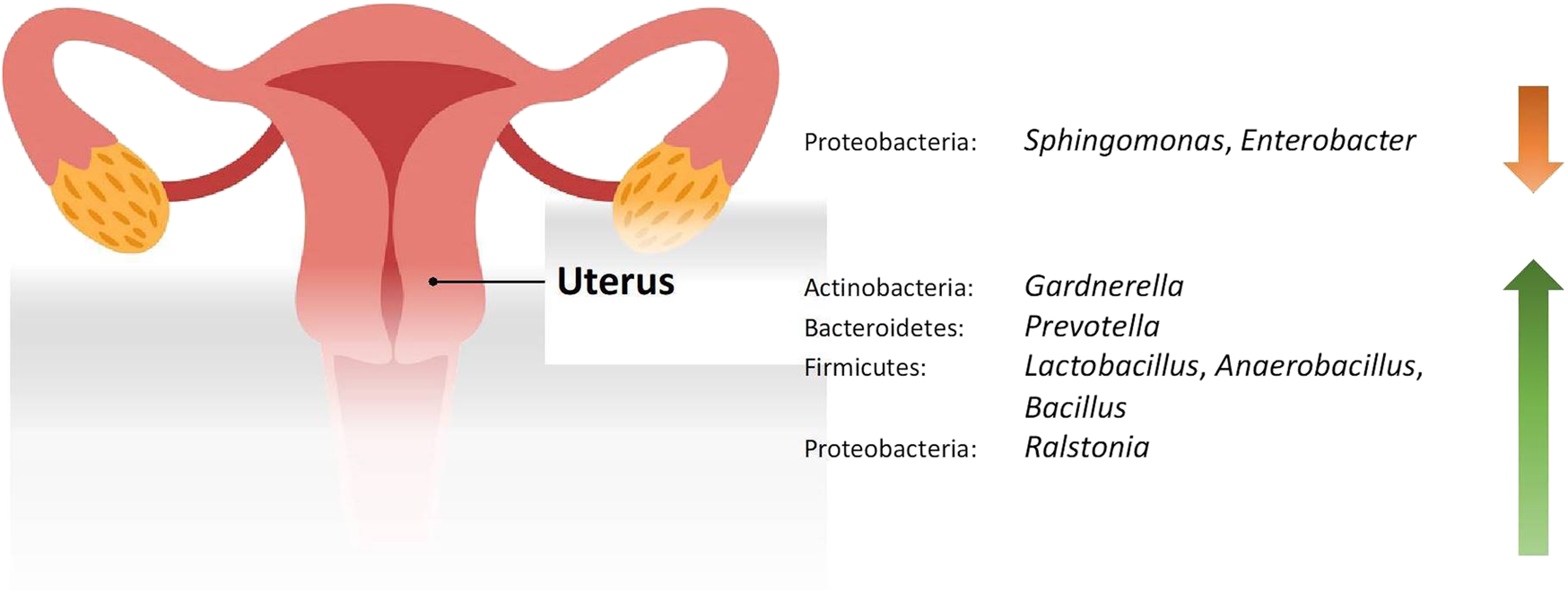
Changes in the relative abundance of the uterine microbiome in reproductive conditions compared to the healthy controls.
The Genital Tract Microbiome Among Healthy, Fertile Women Is Dominated by Lactobacillus
The microbiome of the female reproductive system is best understood when described from the lower to the upper genital tract. All studies in healthy women demonstrated that Lactobacillus dominates the vagina. This Gram-positive rod bacterium provides a major source of vaginal lactic acid by processing glycogen and its byproducts. Human α-amylase catabolises glycogen to maltose, maltotriose, and α-dextrines, which are substrates for Lactobacillus to produce lactic acid. This leads to a low vaginal pH, which is conducive to the growth of Lactobacillus itself. This acidic environment is also essential for the other protective effects of Lactobacillus, such as antimicrobial activity and anti-inflammatory effects [61].
Apart from Lactobacillus, members of the phylum Actinobacteria were also frequently reported, especially Gardnerella, Bifidobacterium, and Atopobium although they only make up a small part of the microbiome. Bifidobacterium is another bacteria genus that might play an important role in the female genital tract. Similar to Lactobacillus, they too confer protection by producing lactic acid and hydrogen peroxide. This prevents the overgrowth of harmful bacteria and helps to maintain the homeostasis of the vaginal microbiome [62]. Gardnerella and Atopobium, on the other hand, are frequently associated with bacterial vaginosis (BV), which is the most prevalent bacterial vaginal infection in women of reproductive age. Although both microorganisms are usually detected as a component of the vaginal microbiome in women with BV, it has been found that the involvement of Atopobium in BV rarely occurs in the absence of Gardnerella. Therefore, it is hypothesised that Atopobium benefits from Gardnerella to survive [63]. Prevotella, a Gram-negative anaerobic bacteria under the phylum Bacteroidetes, is also associated with BV. Similarly, there is also a symbiotic relationship between Gardnerella and Prevotella, whereby the presence of either stimulates the growth of the other [64]. From the phylum Fusobacteria, Sneathia was the only genus identified. This genus of Gram-negative, anaerobic bacteria is also postulated to be involved in the pathogenesis of BV [65]. While these BV-associated organisms exist in the vagina alongside Lactobacillus, they are kept dormant by the protective actions of Lactobacillus as stated earlier. For these reasons, the vaginal microbiota in healthy women would be expected to exhibit lactobacilli dominance [61].
However, the mean relative abundance of Lactobacillus identified in the vagina has a wide range. Kyono et al. [19] found that 99.80% of the vagina was composed of Lactobacillus, but Lin et al. [36] documented that the abundance of Lactobacillus was only 43.88%. This can be due to patient characteristics in the latter study. The healthy controls were negative for BV based on the guidelines of the Infection Disease Society of America, but from the viewpoint of traditional Chinese medicine (TCM), they were classified into either having spleen-deficiency syndrome or damp-heat syndrome. In these classifications, patients displayed distinct symptoms such as leucorrhea and tongue coating, as observed in TCM examinations [36]. The correlation between these syndromes and Western medicine diagnoses is unclear, thus its effect on the vaginal microbiome is unknown.
Lactobacillus was also the most abundant taxon in the cervix of healthy, fertile women, ranging from 64.3% to 96.2%. However, no studies with paired samples from the vagina and cervix compared the abundance of Lactobacillus between both sites. Other bacteria that were identified in the cervix include Gardnerella, Bifidobacterium, Atopobium, Prevotella, and Sneathia. The anatomical continuity can explain the similarity in the microbiome of the vagina and cervix although the cervix is considered a part of the upper genital tract.
Several studies on the microbiome of the endometrium were found. However, only three studies were included in this review by applying the exclusion criteria. There is also a discrepancy between the results of different studies. Moreno et al. [18] and Kyono et al. [19] collected endometrial fluid through aspiration and reported that Lactobacillus dominated the endometrial microbiome. On the other hand, Fang et al. [17] used endometrial swabs and noted that the abundance of Lactobacillus was 6.23%. All three studies also assessed the vaginal microbiota, and the first two revealed that the endometrium and vagina shared similar microbial community composition, while Fang et al. found that the microbial population in the endometrium was quite different from that in the vagina. It is unclear whether this difference is due to different sample collection techniques. In all the sampling procedures, the cervix was first disinfected. Endometrial fluid was aspirated through a trans-cervical catheter, whereas endometrial swabs with sleeves were inserted into the uterine cavity. In both methods, care was taken to avoid contact with the vaginal wall to minimize the risk of contamination. Nevertheless, as sampling was done through the trans-cervical route, there was still a chance of cross-contamination with the cervical microbiota. This makes it hard to ascertain whether Lactobacillus identified in the endometrium ascended from the vagina or if they are true colonisers of the uterine cavity. Samples collected through laparoscopy, laparotomy, or hysterectomy would eliminate this problem. However, gaining consent for this to be carried out in healthy, fertile women is impossible. Therefore, no consensus exists regarding the healthy bacterial microbiome configuration in the endometrium.
To date, there are comparatively few studies assessing the microbiome of the fallopian tube, and only two studies were included in this review. In the study conducted by Pelzer et al. [15], some patients were prescribed oral tinidazole in the evening before surgery. Although antibiotic use was not listed as the exclusion criteria, it can potentially alter the microbiome of the fallopian tube. Tinidazole has antimicrobial actions and is active against protozoa and obligate anaerobic bacteria. Therefore, anaerobes might be under-represented in women who received tinidazole. The study by Zhou et al. (2019) only provided data on phyla level and found that Proteobacteria was the most abundant. Proteobacteria are the largest phylum within the bacteria domain, but other than the common trait of being Gram-negative, no specific morphological or physiological traits characterise the members within each class [66]. As the results from the study are non-specific, they only contribute minimally to our understanding of the microbiome of the fallopian tube.
Despite the lack of studies, it is obvious that the microbiome of the lower genital tract differs significantly from the upper genital tract, with the endometrium likely being a zone of transition. Contrary to the previous belief that the upper genital tract is sterile, it actually harbours its own resident microbiota and represents a distinct ecological niche compared to the lower genital tract [9, 15]. Overall, a trend can be observed along the female genital tract. Lactobacillus is the only genus that was identified in all the genital sites. Its abundance is highest in the vagina, gradually decreasing along the upper genital tract. The difference in pH throughout the female genital tract can explain this. As mentioned above, Lactobacillus thrives in an acidic environment. In general, pH levels are lower in the vagina and cervix compared to the uterus and fallopian tube [67]. For this reason, even if lactobacilli ascends into the upper genital tract, it is unlikely to colonise the site due to unsuitable living conditions.
Tubal Pregnancy and the Genital Tract Microbiome
The fallopian tube is the most common site of ectopic pregnancy; however, no studies conducted were eligible for our scoping study. Nevertheless, studies have postulated several theories on the pathogenesis of ectopic pregnancy. Evolving into an inflammatory environment may potentially be caused by or cause changes in the fallopian tube microbiome [10]. In fact, it is important to note that the fallopian tube microbiome is especially different from the vagina and cervix, which are Lactobacillus-dominant. Therefore, the suitability of the lower genital tract microbiome as a proxy for resembling the fallopian tube condition, microbiota profile, and microenvironment is still a topic for discussion. Currently, insufficient evidence exists regarding potential associations between the microbiome of the upper and lower genital tracts. Few studies have demonstrated a shift from the microbiome of the upper genital tract to that of the lower genital tract, or vice versa. In a recent study, researchers conducted a nested case-control study comparing the vaginal microbiome of women with fallopian tube pregnancy and intrauterine pregnancy in the first trimester. Changes in relative abundances of various taxa were identified in women with fallopian tube pregnancy; specifically, genus Gardnerella, genus Prevotella, class Clostridia, and family Leptotrichiaceae were significantly increased. In contrast, there were no significant changes in the relative abundance of Lactobacillus [47]. The justification for researching the correlation between the microbiomes of the upper and lower genital tracts stems from the practical advantage and feasibility of obtaining samples from the lower genital tract. This is particularly significant if a potential proxy can be identified and utilised as a screening or diagnostic biomarker for reproductive conditions in the future. The exploration of the genital microbiome may pave the way for innovative approaches to reproductive health assessment, offering valuable insights and opportunities for enhanced diagnostics and interventions.
Clinically Significant Pathogens in Ectopic Pregnancy
No doubt, a lot of focus and attention has been given to the Lactobacillus genus and its different species by researchers, as it is the dominant taxa in the lower genital tract of the majority of women. However, it is crucial to note that the various microorganisms do not function individually but instead work as a system. Consequently, it is not only the Lactobacillus genus that matters. Bacteria that are present in minute amounts or very small relative abundances may have great effects or clinical significance. Such observations have been widely reported in other human microbiome research, such as the oral [68] and gut microbiome [69, 70]. A pattern noted is that the “causative organism” in a disease, which is usually cultured or detected by PCR, is not actually present in high relative abundances. For example, a high abundance of genus Chlamydia may be expected in Chlamydia trachomatis (CT) infection, but this is not the case. Several studies have shown relative abundances of Chlamydia of less than 0.1% in both the vaginal and cervical samples [21, 34, 56]. Various studies have described the strong associations between prior CT infections with ectopic pregnancy, where tubal damage was one of the potential mechanisms underlying this correlation [71, 72]. A recent study assessing the presence of chlamydia IgG in women with a confirmed diagnosis of ectopic pregnancy showed that the odds for chlamydia infection were higher compared to normal pregnancies [73]. Additionally, the majority of the cases from this study did not have the classic risk factors associated with ectopic pregnancy which further ascertained the need to explore female reproductive tract dysbiosis as a potential cause.
Current Limitations
There are some limitations to this review. First of all, the number of studies was insufficient, especially those from the upper genital tract, due to the technical and ethical difficulties. Most vaginal samples were collected through a vaginal swab, which is simple to perform. In contrast, fallopian tube samples were collected through dissection following procedures involving salpingectomy, which is invasive. Because of this, it is also harder to recruit healthy subjects other than women who were undergoing salpingectomy for benign conditions, but the effects of these conditions on the microbiome are unknown.
Although all studies utilised next-generation sequencing techniques, they varied in their selection of hypervariable regions to explore the microbiota of the female genital tract, which is a critical factor that significantly influences the depth and precision of microbial community analysis. Sirichoat et al. compared the taxa identified by sequencing the V2, V3, V4, V6-7, V8, and V9 regions of the 16S rRNA gene separately. It was found that each individual region could uniquely identify bacteria taxa that were not identified by other regions. For example, Brevibacterium, Finegoldia, Ruminococcus, and Howardella were only detected by V3 and not the other hypervariable regions although these genera are not significant in regards to the microbiome of the female genital tract [45]. The regions also differed in the number of taxa identified, with the highest being V3, followed by V6-7, V4, V2, V8, and V9. Besides, the same study also found that the results generated by V3 were the most similar compared to those obtained when all regions were sequenced, implying that V3 would be the most accurate representation of the microbiome of the female genital tract [45]. This is another potential cause of incongruence, and the adoption of standardised methodology will facilitate comparison between studies. Furthermore, when handling low biomass samples such as fallopian tubes and endometrial fluid, a negative control should be included in the studies in order to remove potential laboratory contaminants.
In this review, there were a few exclusion criteria for the characteristics of the patients included in the individual studies. Postmenopausal status, use of hormonal contraception, and pregnancy are factors that might change the milieu of the female genital tract, and studies that recruited these patients were excluded. However, some studies did not specify whether the subjects were pre- or post-menopausal, or whether they used hormonal contraception. These studies were included nevertheless but might provide a different result from the other studies that controlled for these parameters.
In conclusion, a general trend in changes in the microbiome profile has been noted, with mainly a reduction of Lactobacillus and an increase in other anaerobic bacteria in the lower genital tract in the disease state. Changes in the upper genital tract are inconclusive and future research with a standardised methodology addressing limitations in our current review can be conducted to determine changes with greater confidence. Researchers should also investigate minor taxa in various reproductive health conditions for their clinical significance. To reiterate, more studies with larger sample sizes, longitudinal studies, collection of data on patient characteristics, standardised sequencing platforms, and hypervariable regions should be considered in the future to achieve valid and convincing results. Finally, microbial metabolomics or shotgun metagenomics [74] can be performed in order to explore functional relationships of the female genital tract microbiota. It will be of immense clinical significance if a proxy can be found.
Statements
Author contributions
Conceptualisation: VA and PY. Literature search and data analysis: HT and CP. Writing–original draft preparation: HT and CP. Critical revision: VA and PY. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/bjbs.2023.12098/full#supplementary-material
References
1.
Punzón-JiménezPLabartaE. The Impact of the Female Genital Tract Microbiome in Women Health and Reproduction: A Review. J Assist Reprod Genet (2021) 38(10):2519–41. 10.1007/s10815-021-02247-5
2.
RavelJGajerPAbdoZSchneiderGMKoenigSSMcCulleSLet alVaginal Microbiome of Reproductive-Age Women. Proc Natl Acad Sci U S A (2011) 108(1):4680–7. 10.1073/pnas.1002611107
3.
QuayleAJ. The Innate and Early Immune Response to Pathogen Challenge in the Female Genital Tract and the Pivotal Role of Epithelial Cells. J Reprod Immunol (2002) 57(1-2):61–79. 10.1016/s0165-0378(02)00019-0
4.
ZervomanolakisIOttHWHadziomerovicDMattleVSeeberBEVirgoliniIet alPhysiology of Upward Transport in the Human Female Genital Tract. Ann N Y Acad Sci (2007) 1101:1–20. 10.1196/annals.1389.032
5.
BakerJMChaseDMHerbst-KralovetzMM. Uterine Microbiota: Residents, Tourists, or Invaders?Front Immunol (2018) 9:208. 10.3389/fimmu.2018.00208
6.
KnightRVrbanacATaylorBCAksenovACallewaertCDebeliusJet alBest Practices for Analysing Microbiomes. Nat Rev Microbiol (2018) 16(7):410–22. 10.1038/s41579-018-0029-9
7.
MitraAMacIntyreDAMahajanVLeeYSSmithAMarchesiJRet alComparison of Vaginal Microbiota Sampling Techniques: Cytobrush Versus Swab. Sci Rep (2017) 7(1):9802. 10.1038/s41598-017-09844-4
8.
SingerMKoedooderRBosMPPoortLSchoenmakersSSavelkoulPHMet alThe Profiling of Microbiota in Vaginal Swab Samples Using 16S rRNA Gene Sequencing and IS-Pro Analysis. BMC Microbiol (2021) 21(1):100. 10.1186/s12866-021-02149-7
9.
PericAWeissJVulliemozNBaudDStojanovM. Bacterial Colonization of the Female Upper Genital Tract. Int J Mol Sci (2019) 20(14):3405. 10.3390/ijms20143405
10.
AguileraLLGarcíaICQuevedoIC. Ectopic Pregnancies and Endometrial Microbiota. Curr Opin Obstet Gynecol (2021) 33(3):202–6. 10.1097/GCO.0000000000000709
11.
CreggerMLenzKLearyELeachRFazleabasAWhiteBet alReproductive Microbiomes: Using the Microbiome as a Novel Diagnostic Tool for Endometriosis. Reprod Immunol Open Access (2017) 2:1–7. 10.21767/2476-1974.100036
12.
ParashiSMoukhahSAshrafiM. Main Risk Factors for Ectopic Pregnancy: A Case-Control Study in a Sample of Iranian Women. Int J Fertil Steril (2014) 8(2):147–54.
13.
HuangCCHuangCCLinSYChangCYLinWCChungCHet alAssociation of Pelvic Inflammatory Disease (PID) With Ectopic Pregnancy and Preterm Labor in Taiwan: A Nationwide Population-Based Retrospective Cohort Study. PLoS ONE (2019) 14(8):e0219351. 10.1371/journal.pone.0219351
14.
MoiniAHosseiniRJahangiriNShivaMAkhoondMR. Risk Factors for Ectopic Pregnancy: A Case-Control Study. J Res Med Sci (2014) 19(9):844–9.
15.
PelzerESWillnerDButtiniMHafnerLMTheodoropoulosCHuygensF. The Fallopian Tube Microbiome: Implications for Reproductive Health. Oncotarget (2018) 9(30):21541–51. 10.18632/oncotarget.25059
16.
ZhouBSunCHuangJXiaMGuoELiNet alThe Biodiversity Composition of Microbiome in Ovarian Carcinoma Patients. Sci Rep (2019) 9(1):1691. 10.1038/s41598-018-38031-2
17.
FangRLChenLXShuWSYaoSZWangSWChenYQ. Barcoded Sequencing Reveals Diverse Intrauterine Microbiomes in Patients Suffering With Endometrial Polyps. Am J Transl Res (2016) 8(3):1581–92.
18.
MorenoICodonerFMVilellaFValbuenaDMartinez-BlanchJFJimenez-AlmazanJet alEvidence That the Endometrial Microbiota Has an Effect on Implantation Success or Failure. Am J Obstet Gynecol (2016) 215(6):684–703. 10.1016/j.ajog.2016.09.075
19.
KyonoKHashimotoTNagaiYSakurabaY. Analysis of Endometrial Microbiota by 16S Ribosomal RNA Gene Sequencing Among Infertile Patients: A Single-Center Pilot Study. Reprod Med Biol (2018) 17(3):297–306. 10.1002/rmb2.12105
20.
FilardoSDi PietroMPorporaMGRecineNFarcomeniALatinoMAet alDiversity of Cervical Microbiota in Asymptomatic Chlamydia trachomatis Genital Infection: A Pilot Study. Front Cel Infect Microbiol (2017) 7:321. 10.3389/fcimb.2017.00321
21.
Di PietroMFilardoSPorporaMGRecineNLatinoMASessaR. HPV/Chlamydia trachomatis Co-Infection: Metagenomic Analysis of Cervical Microbiota in Asymptomatic Women. New Microbiol (2018) 41(1):34–41.
22.
GraspeuntnerSBohlmannMKGillmannKSpeerRKuenzelSMarkHet alMicrobiota-Based Analysis Reveals Specific Bacterial Traits and a Novel Strategy for the Diagnosis of Infectious Infertility. PLoS ONE (2018) 13(1):e0191047. 10.1371/journal.pone.0191047
23.
AtaBYildizSTurkgeldiEBrocalVPDinleyiciECMoyaAet alThe Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota Between Women With Stage 3/4 Endometriosis and Healthy Controls. Sci Rep (2019) 9(1):2204. 10.1038/s41598-019-39700-6
24.
ChornaNRomagueraJGodoy-VitorinoF. Cervicovaginal Microbiome and Urine Metabolome Paired Analysis Reveals Niche Partitioning of the Microbiota in Patients With Human Papilloma Virus Infections. Metabolites (2020) 10(1):36. 10.3390/metabo10010036
25.
TuYZhengGDingGWuYXiJGeYet alComparative Analysis of Lower Genital Tract Microbiome Between PCOS and Healthy Women. Front Physiol (2020) 11:1108. 10.3389/fphys.2020.01108
26.
WeiWZhangXTangHZengLWuR. Microbiota Composition and Distribution Along the Female Reproductive Tract of Women With Endometriosis. Ann Clin Microbiol Antimicrob (2020) 19(1):15. 10.1186/s12941-020-00356-0
27.
QingqingBJieZSongbenQJuanCLeiZMuX. Cervicovaginal Microbiota Dysbiosis Correlates With HPV Persistent Infection. Microb Pathog (2021) 152:104617. 10.1016/j.micpath.2020.104617
28.
HongKHHongSKChoSIRaEHanKHKangSBet alAnalysis of the Vaginal Microbiome by Next-Generation Sequencing and Evaluation of Its Performance as a Clinical Diagnostic Tool in Vaginitis. Ann Lab Med (2016) 36(5):441–9. 10.3343/alm.2016.36.5.441
29.
CampiscianoGFlorianFD'EustacchioAStankovicDRicciGDe SetaFet alSubclinical Alteration of the Cervical-Vaginal Microbiome in Women With Idiopathic Infertility. J Cel Physiol (2017) 232(7):1681–8. 10.1002/jcp.25806
30.
BradleyFBirseKHasselrotKNoël-RomasLIntroiniAWeferHet alThe Vaginal Microbiome Amplifies Sex Hormone-Associated Cyclic Changes in Cervicovaginal Inflammation and Epithelial Barrier Disruption. Am J Reprod Immunol (2018) 80(1):e12863. 10.1111/aji.12863
31.
BrotmanRMShardellMDGajerPFadroshDChangKSilverMIet alAssociation Between the Vaginal Microbiota, Menopause Status, and Signs of Vulvovaginal Atrophy. Menopause (2018) 25(11):1321–30. 10.1097/GME.0000000000001236
32.
ChenHMChangTHLinFMLiangCChiuCMYangTLet alVaginal Microbiome Variances in Sample Groups Categorized by Clinical Criteria of Bacterial Vaginosis. BMC Genomics (2018) 19(10):876. 10.1186/s12864-018-5284-7
33.
MatsumotoAYamagishiYMiyamotoKOkaKTakahashiMMikamoH. Characterization of the Vaginal Microbiota of Japanese Women. Anaerobe (2018) 54:172–7. 10.1016/j.anaerobe.2018.10.001
34.
CeccaraniCFoschiCParolinCD'AntuonoAGaspariVConsolandiCet alDiversity of Vaginal Microbiome and Metabolome During Genital Infections. Sci Rep (2019) 9(1):14095. 10.1038/s41598-019-50410-x
35.
HongXFangSHuangKYinJChenJXuanYet alCharacteristics of the Vaginal Microbiome in Cross-Border Female Sex Workers in China: A Case-Control Study. PeerJ (2019) 7:e8131. 10.7717/peerj.8131
36.
LinWXDuXYangLLChenSYQiuWYWuHWet alDifferences in the Composition of Vaginal Microbiota Between Women Exhibiting Spleen-Deficiency Syndrome and Women With Damp-Heat Syndrome, Two of the Most Common Syndromes of Vaginitis in Traditional Chinese Medicine. Evid Based Complement Alternat Med (2019) 2019:5456379. 10.1155/2019/5456379
37.
LiuZKongYGaoYRenYZhengCDengXet alRevealing the Interaction Between Intrauterine Adhesion and Vaginal Microbiota Using Highthroughput Sequencing. Mol Med Rep (2019) 19(5):4167–74. 10.3892/mmr.2019.10092
38.
XuHZhangXYaoWSunYZhangY. Characterization of the Vaginal Microbiome During Cytolytic Vaginosis Using High-Throughput Sequencing. J Clin Lab Anal (2019) 33(1):e22653. 10.1002/jcla.22653
39.
ZhouYWangLPeiFJiMZhangFSunYet alPatients With LR-HPV Infection Have a Distinct Vaginal Microbiota in Comparison With Healthy Controls. Front Cel Infect Microbiol (2019) 9:294. 10.3389/fcimb.2019.00294
40.
ChenYQiuXWangWLiDWuAHongZet alHuman Papillomavirus Infection and Cervical Intraepithelial Neoplasia Progression Are Associated With Increased Vaginal Microbiome Diversity in a Chinese Cohort. BMC Infect Dis (2020) 20(1):629. 10.1186/s12879-020-05324-9
41.
WangCFanALiHYanYQiWWangYet alVaginal Bacterial Profiles of Aerobic Vaginitis: A Case-Control Study. Diagn Microbiol Infect Dis (2020) 96(4):114981. 10.1016/j.diagmicrobio.2019.114981
42.
WangJXuJHanQChuWLuGChanWYet alChanges in the Vaginal Microbiota Associated With Primary Ovarian Failure. BMC Microbiol (2020) 20(1):230. 10.1186/s12866-020-01918-0
43.
XieYFengYLiWZhanFHuangGHuHet alRevealing the Disturbed Vaginal Micobiota Caused by Cervical Cancer Using High-Throughput Sequencing Technology. Front Cel Infect Microbiol (2020) 10:538336. 10.3389/fcimb.2020.538336
44.
ZhaoCWeiZYangJZhangJYuCYangAet alCharacterization of the Vaginal Microbiome in Women With Infertility and Its Potential Correlation With Hormone Stimulation during In Vitro Fertilization Surgery. mSystems (2020) 5(4):e00450-20. 10.1128/mSystems.00450-20
45.
SirichoatASankuntawNEngchanilCBuppasiriPFaksriKNamwatWet alComparison of Different Hypervariable Regions of 16S rRNA for Taxonomic Profiling of Vaginal Microbiota Using Next-Generation Sequencing. Arch Microbiol (2021) 203(3):1159–66. 10.1007/s00203-020-02114-4
46.
AmatoVPapaleoEPasciutaRViganoPFerrareseRClementiNet alDifferential Composition of Vaginal Microbiome, But Not of Seminal Microbiome, Is Associated With Successful Intrauterine Insemination in Couples With Idiopathic Infertility: A Prospective Observational Study. Open Forum Infect Dis (2020) 7(1):ofz525. 10.1093/ofid/ofz525
47.
RuanXFZhangYXChenSLiuXRZhuFFHuangYXet alNon-Lactobacillus-Dominated Vaginal Microbiota Is Associated With a Tubal Pregnancy in Symptomatic Chinese Women in the Early Stage of Pregnancy: A Nested Case-Control Study. Front Cel Infect Microbiol (2021) 11:659505. 10.3389/fcimb.2021.659505
48.
LozanoFMBernabeuALledoBMoralesRDiazMArandaFIet alCharacterization of the Vaginal and Endometrial Microbiome in Patients With Chronic Endometritis. Eur J Obstet Gynecol Reprod Biol (2021) 263:25–32. 10.1016/j.ejogrb.2021.05.045
49.
LiuXCaoYXieXQinXHeXShiCet alAssociation Between Vaginal Microbiota and Risk of Early Pregnancy Miscarriage. Comp Immunol Microbiol Infect Dis (2021) 77:101669. 10.1016/j.cimid.2021.101669
50.
XuLHuangLLianCXueHLuYChenXet alVaginal Microbiota Diversity of Patients With Embryonic Miscarriage by Using 16S rDNA High-Throughput Sequencing. Int J Genomics (2020) 2020:1764959. 10.1155/2020/1764959
51.
BernabeuALledoBDiazMCLozanoFMRuizVFuentesAet alEffect of the Vaginal Microbiome on the Pregnancy Rate in Women Receiving Assisted Reproductive Treatment. J Assist Reprod Genet (2019) 36(10):2111–9. 10.1007/s10815-019-01564-0
52.
KongYLiuZShangQGaoYLiXZhengCet alThe Disordered Vaginal Microbiota Is a Potential Indicator for a Higher Failure of In Vitro Fertilization. Front Med (2020) 7:217. 10.3389/fmed.2020.00217
53.
RiganelliLIebbaVPiccioniMIlluminatiIBonfiglioGNeroniBet alStructural Variations of Vaginal and Endometrial Microbiota: Hints on Female Infertility. Front Cel Infect Microbiol (2020) 10:350. 10.3389/fcimb.2020.00350
54.
HernandesCSilveiraPRodrigues SereiaAFChristoffAPMendesHValter de OliveiraLFet alMicrobiome Profile of Deep Endometriosis Patients: Comparison of Vaginal Fluid, Endometrium and Lesion. Diagn (2020) 10(3):163. 10.3390/diagnostics10030163
55.
HongXQinPYinJShiYXuanYChenZet alClinical Manifestations of Polycystic Ovary Syndrome and Associations With the Vaginal Microbiome: A Cross-Sectional Based Exploratory Study. Front Endocrinol (2021) 12:662725. 10.3389/fendo.2021.662725
56.
FilardoSDi PietroMTranquilliGLatinoMARecineNPorporaMGet alSelected Immunological Mediators and Cervical Microbial Signatures in Women With Chlamydia trachomatis Infection. mSystems (2019) 4(4):e00094-19. 10.1128/mSystems.00094-19
57.
KwasniewskiWWolun-CholewaMKotarskiJWarcholWKuzmaDKwasniewskaAet alMicrobiota Dysbiosis Is Associated With HPV-Induced Cervical Carcinogenesis. Oncol Lett (2018) 16(6):7035–47. 10.3892/ol.2018.9509
58.
VladislavovnaBVBorisovnaKNOlegovnaBIMikhailovnaSKEvgenievichPDVladimirovichAMet alMicrobiome of the Uterus in Women With Unsuccessful In Vitro Fertilization Attempts. Int J Women's Health Reprod Sci. (2020) 8(4):423–7. 10.15296/ijwhr.2020.68
59.
LiuYKoEYWongKKChenXCheungWCLawTSet alEndometrial Microbiota in Infertile Women With and Without Chronic Endometritis as Diagnosed Using a Quantitative and Reference Range-Based Method. Fertil Steril (2019) 112(4):707–17. 10.1016/j.fertnstert.2019.05.015
60.
KhanKNFujishitaAMasumotoHMutoHKitajimaMMasuzakiHet alMolecular Detection of Intrauterine Microbial Colonization in Women With Endometriosis. Eur J Obstet Gynecol Reprod Biol (2016) 199:69–75. 10.1016/j.ejogrb.2016.01.040
61.
AmabebeEAnumbaDOC. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front Med (2018) 5:181. 10.3389/fmed.2018.00181
62.
FreitasACHillJE. Quantification, Isolation and Characterization of Bifidobacterium From the Vaginal Microbiomes of Reproductive Aged Women. Anaerobe (2017) 47:145–56. 10.1016/j.anaerobe.2017.05.012
63.
CastroJRoscaASCoolsPVaneechoutteMCercaN. Gardnerella Vaginalis Enhances Atopobium Vaginae Viability in an In Vitro Model. Front Cel Infect Microbiol (2020) 10:83. 10.3389/fcimb.2020.00083
64.
RandisTMRatnerAJ. Gardnerella and Prevotella: Co-Conspirators in the Pathogenesis of Bacterial Vaginosis. J Infect Dis (2019) 220(7):1085–8. 10.1093/infdis/jiy705
65.
TheisKRFlorovaVRomeroRBorisovABWintersADGalazJet alSneathia: An Emerging Pathogen in Female Reproductive Disease and Adverse Perinatal Outcomes. Crit Rev Microbiol (2021) 47(4):517–42. 10.1080/1040841X.2021.1905606
66.
RizzattiGLopetusoLRGibiinoGBindaCGasbarriniA. Proteobacteria: A Common Factor in Human Diseases. Biomed Res Int (2017) 2017:9351507. 10.1155/2017/9351507
67.
NgKYBMingelsRMorganHMacklonNCheongY. In Vivo Oxygen, Temperature and pH Dynamics in the Female Reproductive Tract and Their Importance in Human Conception: A Systematic Review. Hum Reprod Update (2018) 24(1):15–34. 10.1093/humupd/dmx028
68.
CaiZLinSHuSZhaoL. Structure and Function of Oral Microbial Community in Periodontitis Based on Integrated Data. Front Cel Infect Microbiol (2021) 11:663756. 10.3389/fcimb.2021.663756
69.
YapPSXChongCWAhmad KamarAYapIKSChooYMLaiNMet alNeonatal Intensive Care Unit (NICU) Exposures Exert a Sustained Influence on the Progression of Gut Microbiota and Metabolome in the First Year of Life. Sci Rep (2021) 11(1):1353. 10.1038/s41598-020-80278-1
70.
PortincasaPBonfrateLVaccaMDe AngelisMFarellaILanzaEet alGut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int J Mol Sci (2022) 23(3):1105. 10.3390/ijms23031105
71.
MpiimaDPWasswa SalongoGLugobeHSsemujjuAMumbere MulisyaOMasindaAet alAssociation Between Prior Chlamydia trachomatis Infection and Ectopic Pregnancy at a Tertiary Care Hospital in South Western Uganda. Obstet Gynecol Int (2018) 2018:4827353. 10.1155/2018/4827353
72.
XiaQWangTXianJSongJQiaoYMuZet alRelation of Chlamydia trachomatis Infections to Ectopic Pregnancy: A Meta-Analysis and Systematic Review. Medicine (Baltimore) (2020) 99(1):e18489. 10.1097/MD.0000000000018489
73.
Thirunavuk ArasooVJMasalamaniMRamadasADominicNALiewDDSiaRWJet alAssociation Between Chlamydial Infection With Ectopic and Full-Term Pregnancies: A Case-Control Study. Trop Med Infect Dis (2022) 7(10):285. 10.3390/tropicalmed7100285
74.
PeabodyMAVan RossumTLoRBrinkmanFSL. Evaluation of Shotgun Metagenomics Sequence Classification Methods Using In Silico and In Vitro Simulated Communities. BMC Bioinformatics (2015) 16(362):363. 10.1186/s12859-015-0788-5
Summary
Keywords
16S rRNA, ectopic pregnancy, fallopian tube, microbiome, female genital
Citation
Teh HE, Pung CK, Arasoo VJT and Yap PSX (2024) A Landscape View of the Female Genital Tract Microbiome in Healthy Controls and Women With Reproductive Health Conditions Associated With Ectopic Pregnancy. Br J Biomed Sci 80:12098. doi: 10.3389/bjbs.2023.12098
Received
23 September 2023
Accepted
18 December 2023
Published
12 January 2024
Volume
80 - 2023
Updates
Copyright
© 2024 Teh, Pung, Arasoo and Yap.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Polly Soo Xi Yap, polly.yap@monash.edu
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.