Abstract
Dystonia is a neurologic disorder characterized by abnormal muscle contractions and postures, which is vastly heterogeneous in its etiologies and clinical manifestations. The role of the basal ganglia in the pathogenesis of dystonia is well known, however, there has been a recent surge of evidence implicating the malfunction of a wide network, including a prominent role of the cerebellum. In this review article, we explore the role of the cerebellum in generating dystonia through multiple lines of basic science and clinical evidence. Neurophysiological, radiological, and pathological findings in various dystonia syndromes implicate an important role of the cerebellum. Dystonia additionally accompanies many known ataxic cerebellar disorders such as spinocerebellar ataxia. Genetic and pharmacologic mouse models of dystonia have demonstrated various degrees of cerebellar pathophysiology. There is emerging evidence supporting cerebellar neuromodulation in the treatment of dystonia. Lastly, we describe cerebellar, cortical, and subcortical motor connections which provide a connectomic basis where the cerebellum may play either a primary or ancillary role in generating dystonia.
Introduction
The role of the basal ganglia in the pathophysiology of dystonia is well-described. Our knowledge of genetic dystonias gives us a glimpse into their heterogeneous underlying pathophysiologies. As an example, of the known monogenetic dystonias, a plurality of conditions involve defects in the dopamine signaling pathway, whose role is extensively studied within the basal ganglia. In contrast, while there are known dopaminergic pathways within the cerebellum, much less is known about their functional significance and contribution to pathology [1]. This may partly explain the preponderance of earlier direct evidence explaining the involvement of the basal ganglia in dystonia. However, it is now well-accepted that other areas of the brain are involved in the genesis, modulation, and sequela of dystonia. In particular there has been recent attention towards the role of the cerebellum. Multiple articles to date have described converging lines of evidence through genetic and pharmacologic models of mice, imaging studies, deep brain stimulation (DBS) studies, microelectrode studies, and transcranial magnetic stimulation (TMS) interventional studies. Although the cerebellum is now well accepted as an etiological culprit in causing dystonia, the precise underpinnings have yet to be fully elucidated.
Table 1 describes cerebellar pathologic findings in autopsy studies of various dystonias. Literature reviews examining focal lesions causing dystonia show that lesions of the basal ganglia are most commonly implicated, although there exist numerous cases of cerebellar lesions causing secondary dystonia [2–4]. Furthermore, dystonia is a known co-existent clinical feature in numerous genetic cerebellar disorders. The prevalence of dystonia has been reported to be 13% in spinocerebellar ataxia type 1 (SCA1), 14% in SCA2, 23% in SCA 3% and 5% in SCA6 in the EUROSCA cohort [5]. Interestingly, longer CAG repeat lengths in SCA3 are more likely associated with dystonia, and presence of dystonia is associated with more severe ataxia in SCA1, 2, three but does not change the rate of ataxia progression [6]. Furthermore, around 100 genes have been linked to both ataxia and dystonia, many of which are involved in synaptic transmission and the nervous system development, including some that play a specific role in cerebellar development [7]. Essential tremor, which is known to be a disorder of cerebellar dysfunction, can coexist with idiopathic dystonia. A recent update on tremor by the Movement Disorder Society (MDS) coined the term “Essential Tremor-Plus” for patients with characteristics of essential tremor and other soft neurological signs such as dystonic posturing [8].
TABLE 1
| Dystonia type | Cerebellar pathologic findings | Extra-cerebellar pathologic findings | Comments | References |
|---|---|---|---|---|
| CD | Loss of Purkinje cells, areas of focal gliosis and torpedo bodies | Substantia nigra with ubiquitin-positive intranuclear inclusions known as Marinesco bodies. | 6 postmortem samples of CD patient compared to 16 age-matched controls | Prudente et al, 2013 [30] |
| DYT-TOR1a | Mild-moderate Purkinje cell depletion. | Mild neuronal loss in the Caudate, Putamen, STN, SN, GP, and thalamus | 2 symptomatic and 5 asymptomatic DYT1 carriers Authors reported no consistent disease specific pathological features and that the findings are most likely related to advanced age at death |
Paudel et al, 2014 [45] |
| DYT-TOR1a | Neuronal hypertrophy of DN in all DYT1 carriers | N/A | 4 symptomatic and 3 asymptomatic DYT1 carriers compared to 5 controls | Iacono et al, 2023 [44] |
| DYT12 -RDP (ATP1A3 mutation) | Mild to moderate neuronal loss/gliosis in Purkinje and granule cell layers, and DN. In 3 siblings (2 symptomatic and 1 asymptomatic carrier), there was swelling of axons of the Purkinje cells (torpedoes |
Neuronal loss/gliosis in GP, STN, periaqueductal gray matter, RN, inferior olivary nucleus | 4 siblings (3 symptomatic, 1 asymptomatic carrier) compared to 16 controls Cerebellar samples were available in only 2 of the symptomatic patients and were compared to the asymptomatic sibling Asymptomatic sibling did not have significant degeneration in cerebellar and extracerebellar regions |
Oblak et al, 2014 [55] |
| Dystonia associated with SCA 1,2, 3, 6, 7 | Variable degrees of Purkinje and deep cerebellar neuronal loss | Widespread neuronal loss of cerebral cortex, basal ganglia, thalamus, brainstem, sensory pathways | SCA 6 neuropathology is confined to the cerebellum | Rub et al, 2013 [61] |
| Ataxia telangiectasia-related dystonia | Severe cerebellar cortical and Purkinje cell loss with less pronounced atrophy of the DN and the inferior olivary nucleus | Dorsal column degeneration and neurogenic muscular atrophy. | Variable phenotypes including focal, multifocal and generalized dystonia | Verhagen et al, 2012 [93] Kuhm et al, 2015 [94] Meneret et al, 2014 [95] |
Cerebellar neuropathology in dystonia.
Abbreviations: CD, cervical dystonia; STN, subthalamic nucleus; SN, substantia nigra; GP, globus pallidus; DN, dentate nucleus; RN, red nucleus; SCA, spinocerebellar ataxia.
Yet if cerebellar dysfunction can cause dystonia, one might ask why most focal lesions of the cerebellum do not. Furthermore, why do only some, but not all, dystonia syndromes show signs of ataxia? In 2014, Prudente and colleagues proposed that most lesions (or similarly, degenerative conditions) cause hypofunction of cerebellum, which lead to the classic syndrome of motor ataxia. Alternatively, lesions or biochemical perturbations causing altered function of the cerebellum may instead generate dystonia [9]. This is in line with the varied clinical signs seen in other neural motor substrates. For instance, lesions of the primary motor cortex causing hypofunction will classically cause contralateral motor weakness, whereas motor cortex hyperexcitability may cause cortical reflex myoclonus or focal motor seizures [10–12]. Motor neuropathy with hypofunction causes weakness, whereas peripheral nerve hyperexcitability leads to cramps, fasciculations, or neuromyotonia [13].
As early as the 1980s, mouse models of dystonia have shown abnormal cerebellar metabolism [14, 15]. In 2002, Pizoli et al. injected kainite (a glutamate agonist; an excitatory neurotransmitter) into the cerebellar vermis in normal mice and found subsequent dystonic behavior. Interestingly, there was no dystonic response when kainite was injected into the basal ganglia or the lateral ventricles [16]. Recent studies have demonstrated abnormal distinct cerebellar firing patterns from Purkinje cells and deep cerebellar nuclei of dystonic mice [17, 18]. The cerebellar output nuclei seem to have differing aberrant spike patterns (especially in regards to rhythmicity and inter-spike interval) between mouse models of dystonia, ataxia, and tremor [19]. Furthermore, when these spiking patterns were replicated in healthy mice, the motor phenotype matched the spiking patterns seen in disease models [19]. This suggests that the abnormal spike patterns seen in cerebellar movement disorders are 1) distinct from each other and 2) not purely a result of plasticity or pathway alterations. Table 2 describes cerebellar dysfunction in various mouse models of dystonia.
TABLE 2
| Mouse model | Disease modeled | Findings | References |
|---|---|---|---|
| Dt Rat – Autosomal recessive mutation of atcay gene leading to caytaxin protein deficiency | Generalized dystonia | Reduced 3′5′ cGMP levels (a biomarker for Purkinje cells) in the cerebellum Abnormal bursting firing patterns in the cerebellar nuclei Cerebellectomy eliminated the dystonic behavior |
LeDoux et al, 1995 [65] LeDoux et al, 1993 [67] Lorden et al, 1985 [15] Xiao et al, 2005 [96] |
| Kainic acid injection into normal mice | Generalized Dystonia | Dystonia in response to kainic acid injection into the cerebellum | Pizoli et al, 2002 [16] |
| CACNA1A Leaner mouse | Ataxia and generalized dystonia | Abnormal cerebellar transmission and pacemaking | Ovsepian et al, 2008 [62] |
| Transgenic mice expressing mutant human torsinA Protein | Generalized dystonia | Increase metabolic demand in the inferior olive and Purkinje cell layer | Zhao et al, 2011 [49] |
| Oubain injection into normal mice | RDP like dystonic behavior | Dystonia in response to Oubain (an ATPase inhibitor) injection either into the cerebellum and basal ganglia or only into the cerebellum When ouabain is injected into only the basal ganglia, mice develop parkinsonism but not dystonia |
Calderon et al, 2011 [54] |
| Dyt1 ΔGAG heterozygous knock-in mice | DYT-TOR1a | Improved motor performance after conditional cerebellar Purkinje knock-out of DYT1 | Yokoi et al, 2013 [66] |
| Oubain injection into normal mice | RDP like dystonic behavior | Dystonia in response to Oubain infusion into the cerebellum Abnormal high frequency erratic bursting firing activity of deep cerebellar nuclei and Purkinje cells The dystonia and abnormal firing activity were reversible after removal of Oubain |
Fremont et al, 2014 [57] |
| Two DYT1 mice strains: heterozygous torsinA knockout mice and human ΔGAG mutant torsinA mice | DYT-TOR1a | Compromised cerebellar synaptogenesis | Vanni et al, 2015 [50] |
| TorsinA knock down mice | DYT-TOR1a | Abnormal bursting firing activity of deep cerebellar nuclei and Purkinje cells TorsinA knockdown in the cerebellum, but not in the basal ganglia, was sufficient to induce dystonia |
Fremont et al, 2017 [51] |
| CACNA1A Mutation in two mice populations: Tottering mouse and Rocker mouse | Paroxysmal dystonia | Paroxysm of dystonia with interictal ataxia Cerebellectomy resulted in resolution of dystonic movements in tottering mice |
Shirley et al, 2018 [63] |
| Loss of Vglut2 leading to elimination of inferior olivary input to cerebellum | Dystonia | Irregular cerebellar firing patterns from Purkinje cells and deep cerebellar nuclei Dystonic behavior improved with cerebellar DBS. |
Brown et al, 2023 [17] White et al, 2017 [97] |
Cerebellar dysfunction in dystonic mice.
Abbreviations: cGMP, cyclic guanosine monophosphate; Vglut2, Vesicular glutamate transporter 2; DBS, deep brain stimulation.
The features of dystonia are vastly heterogeneous. It is because of this heterogeneity that one cannot ascribe dystonia to a singular anatomical defect. Rather, depending on the etiology, there is variable malfunction in different parts of a network which include the basal ganglia, cerebellum, and cortex. In this review, we describe several lines of evidence implicating cerebellar dysfunction as a driving force in disruption of a broader network involved in the pathogenesis of dystonia.
Focal dystonias
Focal dystonias are most commonly adult-onset and favor one group of muscles. They are most commonly idiopathic but may be associated with focal brain lesions or other neurologic conditions. Cervical dystonia (CD) involves sustained or intermittent overactivation of the neck muscles causing a turn, tilt, or tremor of the head and neck. Blepharospasm manifests as increased blink rate and spasms of eye closure. Spasmodic dysphonia involves dystonia of the vocal cords. Focal limb dystonia involves task-specific hand and foot postures. Contiguous body segments may manifest as segmental dystonia, such as Meige syndrome which involves the craniocervical areas.
Lesional cases of focal dystonia are often clinically indistinguishable from idiopathic focal dystonia [2]. A literature review of symptomatic (secondary) cervical dystonia due to focal CNS lesions showed that 11 of 25 contributory lesions were located in the cerebellum, and that all 25 lesions were functionally connected to the cerebellar vermis, dentate nucleus, or cerebellar cortex [2]. Similarly, in secondary blepharospasm, nine of 48 contributory lesions were in the cerebellum [3]. Another review of published cases of lesion-induced dystonia involving over 350 cases showed a correlation between the location of the lesion and the clinical phenotype, with lesions of the brainstem and cerebellum being more often associated with cervical dystonia and blepharospasm compared to lesions involving the basal ganglia and thalamus, which were associated more with limb and hand dystonia, respectively [20].
Patients with focal dystonia demonstrate abnormalities in measures of cerebellar function. For instance, CD and focal limb dystonia patients demonstrate an abnormal eye blink reflex to classical conditioning - a pathway dependent on cerebellar circuits [21]. The cerebellum also plays an important role in motor adaptation. Adaptation of gait is abnormal in blepharospasm and focal hand dystonia, but not in CD [22]. Other the other hand, patients with CD are unable to adapt their saccadic eye movements to varying stimuli [23]. In the syndrome of oculopalatal tremor (OPT), a focal brainstem lesion creates pseudohypertrophy of the inferior olive, which generates maladaptive hypersynchronous cerebellar input and output [24, 25]. These OPT patients are unable to perform saccade adaptations, just as patients with cervical dystonia [23]. It is believed that such oscillations and abnormal saccadic adaptation in cervical dystonia are due to a maladaptive process occurring at the cerebellum [26]. This lends support to the notion that it may be cerebellar maladaptive altered function or hyperactivity, rather than hypofunction, which can cause dystonia.
Further evidence indicates blepharospasm patients have decreased fMRI connectivity from the cerebellum to both somatosensory and visual association cortices [27]. However, a more recent study found increased fMRI connectivity between the dentate nucleus with the bilateral sensorimotor cortices in patients with cervical dystonia and blepharospasm compared to healthy controls, hypothesizing that there is a loss of the normal antecorrelation between the cerebellum and these cortical areas [28]. Despite some variability of the above studies, they all show an overall altered connectivity between the cerebellum and multiple areas believed to be involved in the dystonia network.
Pathologic studies of isolated focal dystonias have largely shown heterogeneous areas of cell loss [29]. However, idiopathic (non-lesional) CD patients have been found to have reduced number of Purkinje cells, increased torpedo bodies (Purkinje axon swellings), and increased cerebellar gliosis compared to controls [30].
Voxel-based morphometry (VBM) uses MRI to compute differences in size of neuroanatomical structures. Using this method, cervical dystonia patients are shown to have increased size of the cerebellar flocculus [31], hemispheres [32], and vermis [33], compared to controls. Patients with focal hand dystonia had decreased size of cerebellar hemispheres compared to controls [34]. A recent meta-analysis of brain abnormalities in patients with idiopathic cervical dystonia revealed significant and diffuse structural (using VBM) and functional imaging changes involving multiple cortical and subcortical areas. With regards to the cerebellum, there was increased gray matter volume in the right hemisphere but decreased volume in the left hemisphere, with increased overall cerebellar activity compared to control. There were no details regarding the laterality of the dystonia [35]. Overall, these studies suggest significant heterogeneity even within specific types of focal dystonia but further demonstrate the metabolic and structural derangements within the cerebellum.
Another way to study the pathogenesis of dystonia is using transcranial magnetic stimulation (TMS). Continuous Theta Burst Stimulation (cTBS) is considered to be an inhibitory form of TMS, whereas intermittent Theta Burst Stimulation (iTBS) is considered to be an excitatory stimulus. In this fashion, the effects of TMS on the cerebellum can be measured in terms of excitability on the contralateral primary motor cortex (M1). Whereas normal subjects have a reduced motor evoked potential (MEP) in response to a cerebellar conditioning stimulus, those with cervical dystonia lack such a response. Both facilitation (using iTBS) and inhibition (using cTBS) of MEP were impaired in cervical dystonia patients compared to controls [36]. This was similarly shown in patients with focal limb dystonia [37]. Remarkably, therapeutic iTBS of the cerebellum, in combination with motor training, seems to improve this maladaptive plasticity in cervical dystonia patients [38]. Both cervical dystonia patients, as well as normal controls whose heads are turned, have opposite directions (compared to neutral position controls) of M1 plasticity in response to cerebellar TMS. In the same study, vibration over the sternocleidomastoid had a similar effect [39].This suggests that abnormal cerebellar processing of proprioceptive inputs may cause dysfunction in cervical dystonia, which is further discussed in the section on neural integration.
DYT-TOR1a
DYT-TOR1a (formerly DYT1) is the most common form of primary generalized dystonia. It is caused by autosomal dominant variant of the TOR1a gene with incomplete penetrance [40]. The exact function of the protein torsin1a is unknown, but it is thought to be involved in cellular transport. It is expressed in the cerebellum, striatum, hippocampus, and substantia nigra [41]. DYT-TOR1a patients are known to have increased metabolism of the cerebellum, thalamus, and midbrain on PET imaging [42]. Disruption of cerebellar cholinergic signaling has also been found in DYT-TOR1a patients [43]. The histopathology is characterized by neuronal hypertrophy of the dentate nucleus [44], mild to moderate Purkinje cell loss in all cases, alongside gliosis of the striatum and substantia nigra [45]. There also appear to be enlarged dopaminergic nigral neurons [46]. The majority of mouse studies show normal gross brain anatomy, but there are microstructural changes in the cerebellum [47, 48] of DYT1 knock-in mice. In another transgenic mouse model, there is an increased energy demand in the cerebellum, but decreased in the basal ganglia [49]. There is also compromised cerebellar synaptogenesis in DYT1 knockout mice [50]. Another model showed that knockdown of torsinA in the cerebellum, but not the basal ganglia, results in generalized dystonia [50]. This occurs in a dose-dependent manner. In the same mice, in vivo recordings of the deep cerebellar nuclei show an erratic abnormal burst pattern compared to a tonic pattern seen in normal mice. Their Purkinje cells also demonstrate abnormal bursting. Knockdown of torsinA additionally disrupts the intrinsic activity (which is independent of synaptic input) of both deep cerebellar nuclei and Purkinje cells [51]. In addition to exhibiting abnormal firing patterns, Purkinje cells in DYT1 knock-in mice demonstrate a reduced peak firing frequency. This reduction is hypothesized to result from an upregulation in both the expression and activity of large conductance calcium-activated potassium (BK) channels within Purkinje neurons [52]. These transmembrane channels modulate neuronal excitability by facilitating membrane hyperpolarization through potassium efflux [53]. Given that Purkinje cells exert inhibitory control over the deep cerebellar nuclei (DCN), a decrease in their firing frequency may lead to disinhibition of the DCN, and ultimately an abnormally enhanced cerebellar outflow. These models implicate an abnormality of the cerebellum as a primary site driving dysfunction in DYT-TOR1a.
Rapid-onset dystonia parkinsonism
Rapid-onset dystonia parkinsonism (RDP, also known as DYT12) is caused by an autosomal dominant variant leading to loss of function of ATP1A3 [54, 55]. Unlike many other primary dystonias, the function of this gene is known - it encodes the alpha3 isoform of the Na-K ATPase. The Na-K pump is expressed throughout the brain, but this isoform is highly expressed in cerebellar Purkinje cells [55]. On histopathology there is mild to moderate neuronal loss and gliosis of Purkinje cells and dentate nucleus, as well as neuronal loss in globus pallidus and STN [55].
When ouabain (an ATPase inhibitor) is injected into the cerebellum and basal ganglia of normal mice, they develop mild motor symptoms which transforms into persistent dystonia when the mice are stressed. Remarkably, when ouabain is injected into only the basal ganglia, those mice become parkinsonian but not dystonic. Yet when it is injected into only the cerebellum, they become dystonic. Furthermore, when the centrolateral nucleus of the thalamus (which relays input from the cerebellum to the striatum) is lesioned, those mice do not become dystonic with ouabain infusion [54]. This suggests that RDP results from a loss of connection from the cerebellum to the basal ganglia. Similar to the DYT1 mouse model, the ouabain RDP mice also demonstrate abnormal high frequency bursting activity in the deep cerebellar nuclei as well as Purkinje cells [56]. Thus, in the dystonias where cerebellar abnormalities are a primary driving force, there may be characteristic bursting patterns, which lends further support towards targeted therapies like TMS.
CACNA1A
CACNA1A encodes a calcium channel subunit. In humans, variants of CACNA1A cause numerous phenotypes including episodic ataxia type 2 (EA2), familial hemiplegic migraine (FHM), and spinocerebellar ataxia type 6 (SCA6) [57]. Most cases with point mutations cause the episodic disorders FHM or EA2, whereas CAG repeat expansions are described to cause the chronic progressive form of SCA6 [57]. Overlap syndromes do exist, and may depend on length of repeat expansion [57]. Additionally, cases have been described in which a variant causes progressive generalized dystonia with mild late ataxia [58], activity-induced dystonia and mild ataxia [59], and episodic ataxia with interictal dystonia [60]. The neuronal toxicity of SCA6 is caused by a calcium channelopathy due to defective α1 subunit of CaV2.1. As a result, SCA6 neuropathology is relatively restricted to the cerebellar cortex [61]. While presence of dystonia was associated with more severe ataxia in SCA 1, 2 and 3, it may predict a slower progression of ataxia in SCA6 [6]. It is possible that the subset of patients with SCA6 and dystonia may exhibit a different compensatory mechanism for loss of Purkinje cells, in which a maladaptive change in physiology protects against ataxia but results in dystonia.
Mouse models of CACNA1A Leaner mice have truncated CACNA1A variants that are used as a model of Purkinje cell neurodegeneration. They develop dystonia at a young age during which there is no Purkinje cell loss but have abnormal cerebellar transmission and pacemaking [62]. However, the dystonic movements improve over time, which paradoxically correlates with decreasing Purkinje cell density over time. This is in line with the notion that cerebellar loss of function (for instance with degenerative cell loss) causes ataxia, whereas altered function may instead cause dystonia [9]. The tottering mouse is a model involving CACNA1A point mutation which exhibits paroxysms of dystonia with interictal ataxia, and the rocker mouse is yet another CACNA1A variant that exhibits a different type of paroxysmal dyskinesia [63]. Notably, in tottering mice, cerebellectomy resulted in resolution of dystonic movements [64]. This is consistent with other studies which have demonstrated improvement following cerebellar lesioning in mice [65–67]. This suggests that with regards to dystonia, absence of cerebellar function may be preferable to malfunction. Overall, the heterogeneity in phenotypes within these mouse models is illustrative of the different cerebellar pathologies that can cause varying degrees of ataxia, dystonia, or both, in the same individual.
Ataxia-telangiectasia
Ataxia-telangiectasia (AT) is an autosomal recessive disorder caused by a variant of the ATM gene, which plays roles in DNA repair and apoptosis. It is a multisystem disorder which principally affects the cerebellum and brainstem [68]. On pathology, there is severe cerebellar atrophy, in addition to atrophy of the inferior olives and dentate nuclei. The most common initial manifestation is ataxia, however 89% of patients develop dystonia over the course of the disease, which can be focal or generalized [68]. The number and severity of manifestations seem to be related to the level of ATM kinase activity [68]. Yet dystonia-predominant forms of AT tend to present with a lower incidence of ataxia and cerebellar atrophy compared with classic forms of the disease [69]. This further illustrates that it may be abnormal cerebellar function, rather than loss of function, which can generate dystonia.
Role of the cerebellum in network dysfunction
Cerebellar function is not universally affected in dystonia. For instance, when measured by eye blink classical conditioning, the cerebellum is dysfunctional in cases of primary but not secondary dystonia [70]. Its involvement in neuroimaging and neuropathologic studies is also inconsistent, yet cerebellar dysfunction clearly plays a direct role in some dystonias and an indirect role in others.
The basal ganglia and cerebellum have previously been considered two distinct subcortical systems performing distinct functions. It was thought that the basal ganglia and cerebellum only communicate through higher order cortical relay, and thereby function independently by relaying projections to cortical areas via separate thalamic nuclei. The cerebello-thalamo-cortical connection which is vital for motor learning and coordination is well-described, yet more recent studies have demonstrated direct connections between the cerebellum and basal ganglia. Recent research has proved that there is a disynaptic connection from the motor subthalamic nucleus (STN), via pontine nuclei, to the cerebellar cortex [71, 72]. Additionally, the cerebellar dentate nucleus has a disynaptic connection, via the intralaminar nucleus of the thalamus, directly to the striatum [72]. The latter synapse is disproportionately connected to the external over the internal segment of the pallidum, which suggests this circuit may have more influence on the indirect pathway [73]. Figure 1 summarizes many of these connections. Taken together, these findings show the existence of a bidirectional connection between the cerebellum and basal ganglia and provide evidence towards a tight network system involving the basal ganglia, cerebellum, and cerebral cortex. It is hypothesized that these pathways may be involved in normal motor learning and adaptation [73]. In disease states like dystonia, abnormal activity at one node can directly lead to a maladaptive response downstream.
FIGURE 1
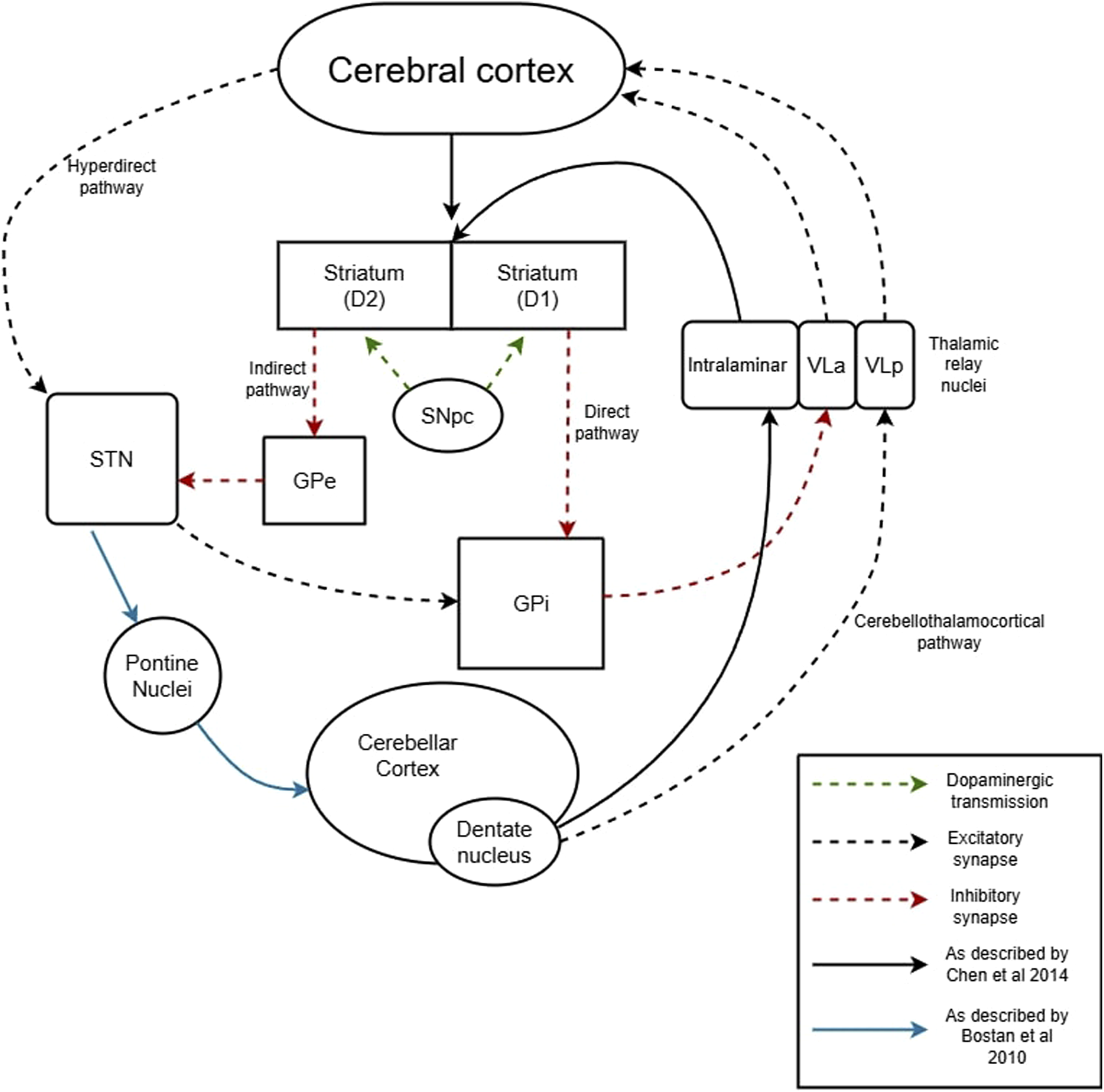
New evidence shows disynaptic connections between STN to cerebellar cortex, and dentate nucleus to striatum. These short-latency pathways provide a framework for a more closely interconnected motor circuit which may play roles in motor adaptation and learning. In dystonia, there is network dysfunction of both basal ganglia and cerebellum. Hyperactivity at one node could conceivably generate a positive-feedback loop which propagate maladaptive postures seen in dystonia.
In idiopathic dystonia, there is overactivity in the STN [74] and cerebellum. In the context of the above model, perhaps some dystonias result from overactivity of one of the structures, with a resultant positive feedback loop propagating the overactivity. Other authors suggest the STN hyperactivity leads to deficient external pallidal “surround inhibition” in the indirect pathway which causes the overflow movements seen in dystonia [74]. Some dystonias may result from altered function of the cerebellum, possibly related to dystonia-specific spike signatures [9, 19]. Yet in others like RDP, the loss of function in the cerebellum may directly generate dystonia likely through its interaction with the basal ganglia.
In our attempt to conceptualize the large quantities of pathological, imaging, and mouse data gathered to date, we are often left with more questions than answers. Why do some focal lesions of the cerebellum and its outflow tracts cause ataxia, while others cause dystonia, and still others cause both? Why are there conflicting reports of both increased and decreased cerebellar volume sizes in the same idiopathic dystonic disease states? Why do CACNA1A Leaner mice improve over time with regards to dystonia, and yet their Purkinje cells progressively degenerate? The precise mechanisms of pathogenicity within this basal ganglia, cerebellar, and cortical network system do not appear to be a universal rule, but rather dependent on the functional nuances of the underlying disorder. The distinction between hypofunction and “altered” function of the cerebellum may be the culprit in heterogenous genetic conditions such as CACNA1A and ataxia telangiectasia, or in lesional cases of focal dystonia. In other conditions such as RDP, a functional disconnection syndrome may exist where the loss of communication between the cerebellum and other motor centers may be the driving force. There remains a possibility of yet unknown variables which predispose individuals to developing dystonia in response to cerebellar pathology.
Cerebellar input into neural integration
In 2002, a series of experiments in macaques utilizing stimulation and inactivation of the midbrain interstitial nucleus of Cajal (INC) implicated this nucleus as a neural integrator of head positioning in the torsional plane [75]. In these experiments, inactivating and stimulating the INC created opposite directions of laterocollis and dystonic head tremor. The authors further posited that in cervical dystonia, there is either intrinsic dysfunction or an imbalance of inputs into the head positioning neural integrators. This is in line with the phenomenology of tremor-predominant cervical dystonia, which produces head drifts towards an abnormal null point and quick jerks towards an intended target. Cervical dystonia is unique amongst dystonias in that there are many physiologic inputs which, when disturbed, can alter one’s sense of head-on-body position and thereby generate abnormal postures. The cerebellum provides one source of feedback to the neural integrator of head position which serves to maintain postures. The other two major inputs are the visual system and neck proprioception [76]. Subconscious proprioceptive information is also relayed through the cerebellum providing further feedback towards head position [77]. Malfunction of any of these systems, including cerebellum, can affect the functioning of the head neural integrator and lead to pathologic postures.
This newer neural integrator framework may also suggest novel avenues for treatment targets. For instance, cerebellar TMS may possibly exert some of its effect via disruption of pathologic proprioceptive integration [39]. Additionally, the framework remains compatible with existing views on the physiology of cervical dystonia because it highlights the same anatomical substrates playing roles in a feedback system. Large lesional studies of patients with secondary focal dystonias suggest that there may be separate networks underlying specific dystonic phenotypes [78]. Cervical dystonia patients are more likely to have lesions of the cerebellum or brainstem compared to other focal dystonias, which supports the neural integrator model in cervical dystonia. In contrast, focal limb dystonia patients are more likely to have lesions within the basal ganglia [78].
Neuromodulation of the cerebellum in the treatment of dystonia
Given the recent evidence suggesting that the basal ganglia, cerebellum and cerebral cortex function as nodes within an integrated network, there has been a growing interest in application of cerebellar DBS to treat dystonia. Stereotactic surgery of the cerebellum was an early treatment modality to address other motor disorders such as spasticity and chorea. The first surgical procedure of the dentate nucleus was carried out in 1935 [79]. In the early 1970s, Irving Cooper implanted electrodes to stimulate the anterior lobe of the cerebellum for cerebral palsy as well as intractable epilepsy [80]. The procedures were largely abandoned when they were replaced by treatments such as intrathecal baclofen and botulinum toxin injections, but interest has now re-emerged. Deep Anterior Cerebellar Stimulation (DACS) is a form of DBS therapy targeting the bilateral anterior cerebellar lobes, and is being used for spasticity due to cerebral palsy. Given that dystonia often co-exists with spasticity in these individuals, improvement in both focal and segmental secondary dystonia has been reported with DACS [81]. There are many reports of motor improvement following cerebellar DBS in dystonic mice models [17, 82]. One study demonstrated improvement that was objectively measured using EMG recording of neck muscle activity [83].
Few case reports have been reported in humans utilizing cerebellar DBS. Table 3 summarizes reports on cerebellar neuromodulation in dystonia patients. Horisawa and colleagues reported a robust response to dentate nucleus DBS in a patient with severe generalized fixed dystonia refractory to bilateral pallidotomy and intrathecal baclofen [84]. The same group later reported a case of improvement in both tremor and dystonia in a patient who underwent palliative cerebellar DBS after an infection necessitated removal of his basal ganglia leads [85]. Similarly, Ostrem et al reported a patient with hemi-dystonia secondary to ischemic injuries to bilateral basal ganglia and brainstem. Two thalamotomies failed to offer benefit, and her lesioned brain tissue was not amenable to traditional DBS targets. Therefore, bilateral dentate nuclei were targeted with a resultant 40% improvement on Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS) which was sustained at 24 months [86]. Lastly, at least six patients with dyskinetic cerebral palsy and resultant dystonia have been reported to benefit from DBS targeting the cerebellum or its outflow tracts [87–90]. An ongoing trial seeks to evaluate dentate nucleus DBS for treatment of dyskinetic cerebral palsy in ten young patients [91]. Notably, cerebellar DBS has mainly been applied thus far in the absence of traditional DBS target availability. Therefore, the benefit of cerebellar stimulation in some of these cases may be in tandem with pre-existing basal ganglionic lesions. As we further advance our understanding of these subcortical circuits and patterns of altered connectivity in specific disease states, newer and more specific cerebellar stimulation targets may be explored.
TABLE 3
| Condition | Modality | Target | Outcome | Comments | References |
|---|---|---|---|---|---|
| CD | cTBS | Bilateral cerebellar hemispheres | ∼15% improvement in TWSTRS at week 2 No significant difference in BFMDRS-MS. |
Double blinded, placebo- controlled trial. 20 patients included 2 weeks of stimulation |
Koch et al, 2014 [92] |
| CP with dystonia | DBS | Bilateral deep anterior lobes of the cerebellum | ∼42% reduction in median total UDRS at the end of follow-up | Retrospective review of 10 CP patients The median time of follow up was 5.5 years |
Sokal et al, 2015 [81] |
| CD | iTBS | Bilateral cerebellar hemispheres | ∼39% improvement in TWSTRS at day 10 | Double blinded, placebo- controlled trial. 10 patients total were included 10 days of stimulation |
Bradnam et al, 2016 [38] |
| Generalized idiopathic dystonia | DBS | Bilateral SCPs and DNs | ∼40% reduction in BADS at 6 months follow up | Case report, previously failed to respond to bilateral pallidotomy and intrathecal baclofen | Horisawa et al, 2019 [84] |
| Acquired hemi-dystonia | DBS | Bilateral cerebellar hemispheres | ∼40% reduction in BFMDRS-MS, at 2 years follow up | Case report, prior two thalamotomies with transient benefits | Brown et al, 2020 [86] |
| CP with dystonia | High frequency DBS | Bilateral SCPs and DNs | ∼36% reduction in BFMDRS-MS at 6 months follow-up | Case report, failed bilateral GPi DBS. DN stimulation needed stronger stimulation intensity and was accompanied by several side effects |
Lin et al, 2020 [88] |
| Multifocal idiopathic dystonia and tremor | DBS | Bilateral SCPs and DNs | ∼92% improvement in BFMDRS-MS and complete resolution of the tremor at 6 months follow up | Case report, infection necessitated removal of BG leads and placement of palliative cerebellar leads | Horisawa et al, 2021 [85] |
| Acquired dystonia | DBS | Cerebellar cortex | ∼70% reduction in BFMDRS-MS | Case report | Stroud et al, 2022 [90] |
| CP with dystonia | DBS | Bilateral SCPs and DNs | 19%–40% reduction in BFMDRS-MS at 2–3 months follow up | Case report, 3 patients included | Cajigas et al, 2023 [87] |
| CP with dystonia | DBS | Bilateral SCP | 30% reduction of BFMDRS-MS at 12 months follow up | Case report, 5 patients included | Lin et al, 2024 [89] |
Case reports of cerebellar modulation for treatment of dystonia.
Abbreviation: CD, cervical dystonia; CP, cerebral palsy; cTBS, continuous theta burst stimulation; iTBS, intermittent theta burst stimulation; DBS, deep brain stimulation; TWSTRS, Toronto Western spasmodic torticollis rating scale; BFMDRS-MS, Burke–Fahn–Marsden Dystonia Rating Scale-Movement Scale; UDRS, unified dystonia rating scale; BADS, Barry-Albright Dystonia Rating Scale; BG, basal ganglia; GPi, Globus pallidus internus; SCP, superior cerebellar peduncle; DN, dentate nucleus.
As previously noted, cerebellar TMS has been used to study the mechanism and involvement of the cerebellum in dystonia. Therapeutic data is more limited, however continuous theta burst (cTBS) showed a modest 15% improvement in the Toronto Western Cervical Dystonia Rating Scale (TWSTRS) scale after 2 weeks of stimulation [92].
Limitations and future directions
The reader should keep in mind the strengths and limitations of study methods described. The advantage of rodent studies is that it allows the use of precise interventions and control of confounders which are not available in human studies. Rodents are inexpensive and have neurobiological similarities to humans, yet there is some argument in rodent models as to whether the induced postures are sufficient to be labelled dystonia, or whether rodents are capable of manifesting dystonia in the first place. Some labs have conducted detailed investigations with electrophysiologic correlates to validate their findings of dystonia in rodents [54].
In humans, advanced imaging modalities like VBM and functional MRI are useful to localize brain abnormalities and abnormal connectivities. However they are limited in that they do not differentiate between causality, secondary changes, and epiphenomena. Similarly, behavioral studies looking at cerebellar function in dystonia patients, or vice versa, can show correlation but not causality.
Interventional studies such as using TMS or DBS can be attractive, as they can help establish direct effect. With the advent of microelectrode and local field potential recordings, more sophisticated studies can be designed to elucidate the effects of neurostimulation on cerebellar physiology. Moreover, the emerging body of evidence in the role of the cerebellum in dystonia has implications in therapies, particularly in novel methods of cerebellar stimulation.
Statements
Author contributions
All authors participated in the review of literature, and manuscript review; AW co-wrote the original draft and created the figure, IA co-wrote the original draft and created the tables, CK participated in manuscript editing and research conception. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
Flace P Livrea P Basile GA Galletta D Bizzoca A Gennarini G et al The cerebellar dopaminergic system. Front Syst Neurosci (2021) 15:650614. 10.3389/fnsys.2021.650614
2.
LeDoux MS Brady KA . Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord (2003) 18(1):60–9. 10.1002/mds.10301
3.
Khooshnoodi MA Factor SA Jinnah HA . Secondary blepharospasm associated with structural lesions of the brain. J Neurol Sci (2013) 331(1-2):98–101. 10.1016/j.jns.2013.05.022
4.
Obeso JA Giménez-Roldán S . Clinicopathological correlation in symptomatic dystonia. Adv Neurol (1988) 50:113–22.
5.
Schmitz-Hubsch T Coudert M Bauer P Giunti P Globas C Baliko L et al Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms. Neurology (2008) 71(13):982–9. 10.1212/01.wnl.0000325057.33666.72
6.
Kuo PH Gan SR Wang J Lo RY Figueroa KP Tomishon D et al Dystonia and ataxia progression in spinocerebellar ataxias. Parkinsonism Relat Disord (2017) 45:75–80. 10.1016/j.parkreldis.2017.10.007
7.
Nibbeling EA Delnooz CC de Koning TJ Sinke RJ Jinnah HA Tijssen MA et al Using the shared genetics of dystonia and ataxia to unravel their pathogenesis. Neurosci Biobehav Rev (2017) 75:22–39. 10.1016/j.neubiorev.2017.01.033
8.
Bhatia KP Bain P Bajaj N Elble RJ Hallett M Louis ED et al Consensus statement on the classification of tremors. From the task force on tremor of the international Parkinson and movement disorder society. Mov Disord (2018) 33(1):75–87. 10.1002/mds.27121
9.
Prudente CN Hess EJ Jinnah HA . Dystonia as a network disorder: what is the role of the cerebellum?Neuroscience (2014) 260:23–35. 10.1016/j.neuroscience.2013.11.062
10.
Shibasaki H Hallett M . Electrophysiological studies of myoclonus. Muscle Nerve (2005) 31(2):157–74. 10.1002/mus.20234
11.
Canafoglia L Meletti S Bisulli F Alvisi L Assenza G d'Orsi G et al A Reappraisal on cortical myoclonus and brief Remarks on myoclonus of different Origins. Clin Neurophysiol Pract (2024) 9:266–78. 10.1016/j.cnp.2024.10.001
12.
Cowan JM Rothwell JC Wise RJ Marsden CD . Electrophysiological and positron emission studies in a patient with cortical myoclonus, epilepsia partialis continua and motor epilepsy. J Neurol Neurosurg Psychiatry (1986) 49(7):796–807. 10.1136/jnnp.49.7.796
13.
Katirji B . Peripheral nerve hyperexcitability. Handb Clin Neurol (2019) 161:281–90. 10.1016/B978-0-444-64142-7.00054-0
14.
Riker DK Messer A Roth RH . Increased noradrenergic metabolism in the cerebellum of the mouse mutant dystonia musculorum. J Neurochem (1981) 37(3):649–54. 10.1111/j.1471-4159.1982.tb12536.x
15.
Lorden JF Oltmans GA McKeon TW Lutes J Beales M . Decreased cerebellar 3',5'-cyclic guanosine monophosphate levels and insensitivity to harmaline in the genetically dystonic rat (dt). J Neurosci (1985) 5(10):2618–25. 10.1523/JNEUROSCI.05-10-02618.1985
16.
Pizoli CE Jinnah HA Billingsley ML Hess EJ . Abnormal cerebellar signaling induces dystonia in mice. J Neurosci (2002) 22(17):7825–33. 10.1523/JNEUROSCI.22-17-07825.2002
17.
Brown AM van der Heijden ME Jinnah HA Sillitoe RV . Cerebellar dysfunction as a source of dystonic phenotypes in mice. Cerebellum (2023) 22(4):719–29. 10.1007/s12311-022-01441-0
18.
van der Heijden ME Sillitoe RV . Cerebellar dysfunction in rodent models with dystonia, tremor, and ataxia. Dystonia (2023) 2:11515. 10.3389/dyst.2023.11515
19.
van der Heijden ME Brown AM Kizek DJ Sillitoe RV . Cerebellar nuclei cells produce distinct pathogenic spike signatures in mouse models of ataxia, dystonia, and tremor. Elife (2024) 12. 10.7554/eLife.91483
20.
Corp DT Greenwood CJ Morrison-Ham J Pullinen J McDowall GM Younger EFP et al Clinical and structural findings in patients with lesion-induced dystonia: descriptive and quantitative analysis of published cases. Neurology (2022) 99(18):e1957–e1967. 10.1212/WNL.0000000000201042
21.
Antelmi E Di Stasio F Rocchi L Erro R Liguori R Ganos C et al Impaired eye blink classical conditioning distinguishes dystonic patients with and without tremor. Parkinsonism Relat Disord (2016) 31:23–7. 10.1016/j.parkreldis.2016.06.011
22.
Hoffland BS Veugen LC Janssen MM Pasman JW Weerdesteyn V van de Warrenburg BP . A gait paradigm reveals different patterns of abnormal cerebellar motor learning in primary focal dystonias. Cerebellum (2014) 13(6):760–6. 10.1007/s12311-014-0594-z
23.
Mahajan A Gupta P Jacobs J Marsili L Sturchio A Jinnah HA et al Impaired saccade adaptation in tremor-dominant cervical dystonia-evidence for maladaptive cerebellum. Cerebellum (2020) 20:678–86. 10.1007/s12311-020-01104-y
24.
Ruigrok TJ de Zeeuw CI Voogd J . Hypertrophy of inferior olivary neurons: a degenerative, regenerative or plasticity phenomenon. Eur J Morphol (1990) 28(2-4):224–39.
25.
Elkasaby M Beylergil SB Gupta P Mahajan A Ghasia FF Shaikh AG . Lessons learned from the syndrome of oculopalatal tremor. J Comput Neurosci (2020) 49:309–18. 10.1007/s10827-020-00757-2
26.
Shaikh AG Ghasia FF DeLong MR Jinnah HA Freeman A Factor SA . Ocular palatal tremor plus dystonia - new syndromic association. Mov Disord Clin Pract (2015) 2(3):267–70. 10.1002/mdc3.12193
27.
Jochim A Li Y Gora-Stahlberg G Mantel T Berndt M Castrop F et al Altered functional connectivity in blepharospasm/orofacial dystonia. Brain Behav (2018) 8(1):e00894. 10.1002/brb3.894
28.
Gianni C Pasqua G Ferrazzano G Tommasin S De Bartolo MI Petsas N et al Focal dystonia: functional connectivity changes in cerebellar-basal ganglia-cortical circuit and preserved global functional architecture. Neurology (2022) 98(14):e1499–e1509. 10.1212/WNL.0000000000200022
29.
Sharma N . Neuropathology of dystonia. Tremor Other Hyperkinet Mov (N Y) (2019) 9:569. 10.7916/d8-j6sx-b156
30.
Prudente CN Pardo CA Xiao J Hanfelt J Hess EJ Ledoux MS et al Neuropathology of cervical dystonia. Exp Neurol (2013) 241:95–104. 10.1016/j.expneurol.2012.11.019
31.
Draganski B Thun-Hohenstein C Bogdahn U Winkler J May A . Motor circuit gray matter changes in idiopathic cervical dystonia. Neurology (2003) 61(9):1228–31. 10.1212/01.wnl.0000094240.93745.83
32.
Obermann M Yaldizli O De Greiff A Lachenmayer ML Buhl AR Tumczak F et al Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord (2007) 22(8):1117–23. 10.1002/mds.21495
33.
Filip P Gallea C Lehéricy S Bertasi E Popa T Mareček R et al Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov Disord (2017) 32(5):757–68. 10.1002/mds.26930
34.
Delmaire C Vidailhet M Elbaz A Bourdain F Bleton JP Sangla S et al Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology (2007) 69(4):376–80. 10.1212/01.wnl.0000266591.49624.1a
35.
Huang X Zhang M Li B Shang H Yang J . Structural and functional brain abnormalities in idiopathic cervical dystonia: a multimodal meta-analysis. Parkinsonism Relat Disord (2022) 103:153–65. 10.1016/j.parkreldis.2022.08.029
36.
Porcacchia P Álvarez de Toledo P Rodríguez-Baena A Martín-Rodríguez JF Palomar FJ Vargas-González L et al Abnormal cerebellar connectivity and plasticity in isolated cervical dystonia. PloS one (2019) 14(1):e0211367. 10.1371/journal.pone.0211367
37.
Brighina F Romano M Giglia G Saia V Puma A Giglia F et al Effects of cerebellar TMS on motor cortex of patients with focal dystonia: a preliminary report. Exp Brain Res (2009) 192(4):651–6. 10.1007/s00221-008-1572-9
38.
Bradnam LV McDonnell MN Ridding MC . Cerebellar intermittent theta-burst stimulation and motor control training in individuals with cervical dystonia. Brain Sci (2016) 6(4):56. 10.3390/brainsci6040056
39.
Popa T Hubsch C James P Richard A Russo M Pradeep S et al Abnormal cerebellar processing of the neck proprioceptive information drives dysfunctions in cervical dystonia. Scientific Rep (2018) 8(1):2263. 10.1038/s41598-018-20510-1
40.
Opal P Tintner R Jankovic J Leung J Breakefield XO Friedman J et al Intrafamilial phenotypic variability of the DYT1 dystonia: from asymptomatic TOR1A gene carrier status to dystonic storm. Mov Disord (2002) 17(2):339–45. 10.1002/mds.10096
41.
Konakova M Huynh DP Yong W Pulst SM . Cellular distribution of torsin A and torsin B in normal human brain. Arch Neurol (2001) 58(6):921–7. 10.1001/archneur.58.6.921
42.
Eidelberg D Moeller JR Antonini A Kazumata K Nakamura T Dhawan V et al Functional brain networks in DYT1 dystonia. Ann Neurol (1998) 44(3):303–12. 10.1002/ana.410440304
43.
Mazere J Dilharreguy B Catheline G Vidailhet M Deffains M Vimont D et al Striatal and cerebellar vesicular acetylcholine transporter expression is disrupted in human DYT1 dystonia. Brain (2021) 144(3):909–23. 10.1093/brain/awaa465
44.
Iacono D Peng H Rabin ML Kurlan R . Neuropathology and morphometry of dentate nucleus neurons in DYT1 brains: cerebellar abnormalities in isolated dystonia. J Neuropathol Exp Neurol (2023) 82(8):695–706. 10.1093/jnen/nlad044
45.
Paudel R Kiely A Li A Lashley T Bandopadhyay R Hardy J et al Neuropathological features of genetically confirmed DYT1 dystonia: investigating disease-specific inclusions. Acta Neuropathologica Commun (2014) 2(1):159. 10.1186/s40478-014-0159-x
46.
Rostasy K Augood SJ Hewett JW Leung JC Sasaki H Ozelius LJ et al TorsinA protein and neuropathology in early onset generalized dystonia with GAG deletion. Neurobiol Dis (2003) 12(1):11–24. 10.1016/s0969-9961(02)00010-4
47.
Song CH Bernhard D Hess EJ Jinnah HA . Subtle microstructural changes of the cerebellum in a knock-in mouse model of DYT1 dystonia. Neurobiol Dis (2014) 62:372–80. 10.1016/j.nbd.2013.10.003
48.
Zhang L Yokoi F Jin YH DeAndrade MP Hashimoto K Standaert DG et al Altered dendritic morphology of Purkinje cells in Dyt1 ΔGAG knock-in and purkinje cell-specific Dyt1 conditional knockout mice. PloS one (2011) 6(3):e18357. 10.1371/journal.pone.0018357
49.
Zhao Y Sharma N LeDoux MS . The DYT1 carrier state increases energy demand in the olivocerebellar network. Neuroscience (2011) 177:183–94. 10.1016/j.neuroscience.2011.01.015
50.
Vanni V Puglisi F Bonsi P Ponterio G Maltese M Pisani A et al Cerebellar synaptogenesis is compromised in mouse models of DYT1 dystonia. Exp Neurol (2015) 271:457–67. 10.1016/j.expneurol.2015.07.005
51.
Fremont R Tewari A Angueyra C Khodakhah K . A role for cerebellum in the hereditary dystonia DYT1. Elife (2017) 6:e22775. 10.7554/eLife.22775
52.
Liu Y Xing H Wilkes BJ Yokoi F Chen H Vaillancourt DE et al The abnormal firing of Purkinje cells in the knockin mouse model of DYT1 dystonia. Brain Res Bull (2020) 165:14–22. 10.1016/j.brainresbull.2020.09.011
53.
Echeverria F Gonzalez-Sanabria N Alvarado-Sanchez R Fernandez M Castillo K Latorre R . Large conductance voltage-and calcium-activated K(+) (BK) channel in health and disease. Front Pharmacol (2024) 15:1373507. 10.3389/fphar.2024.1373507
54.
Calderon DP Fremont R Kraenzlin F Khodakhah K . The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat Neurosci (2011) 14(3):357–65. 10.1038/nn.2753
55.
Oblak AL Hagen MC Sweadner KJ Haq I Whitlow CT Maldjian JA et al Rapid-onset dystonia-parkinsonism associated with the I758S mutation of the ATP1A3 gene: a neuropathologic and neuroanatomical study of four siblings. Acta Neuropathol (2014) 128(1):81–98. 10.1007/s00401-014-1279-x
56.
Fremont R Calderon DP Maleki S Khodakhah K . Abnormal high-frequency burst firing of cerebellar neurons in rapid-onset dystonia-parkinsonism. J Neurosci (2014) 34(35):11723–32. 10.1523/JNEUROSCI.1409-14.2014
57.
Jodice C Mantuano E Veneziano L Trettel F Sabbadini G Calandriello L et al Episodic ataxia type 2 (EA2) and spinocerebellar ataxia type 6 (SCA6) due to CAG repeat expansion in the CACNA1A gene on chromosome 19p. Hum Mol Genet (1997) 6(11):1973–8. 10.1093/hmg/6.11.1973
58.
Rinaldi D Tangari MM Ledda C Dematteis F Rizzone MG Lopiano L et al CACNA1A variant associated with generalized dystonia. Neurol Sci (2024) 45(9):4589–92. 10.1007/s10072-024-07592-8
59.
Stampfl B Fee D . Novel mutation in CACNA1A associated with activity-induced dystonia, cervical dystonia, and mild ataxia. Case Rep Neurol Med (2021) 2021:7797770. 10.1155/2021/7797770
60.
Spacey SD Materek LA Szczygielski BI Bird TD . Two novel CACNA1A gene mutations associated with episodic ataxia type 2 and interictal dystonia. Arch Neurol (2005) 62(2):314–6. 10.1001/archneur.62.2.314
61.
Rub U Schols L Paulson H Auburger G Kermer P Jen JC et al Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog Neurobiol (2013) 104:38–66. 10.1016/j.pneurobio.2013.01.001
62.
Ovsepian SV Friel DD . The leaner P/Q-type calcium channel mutation renders cerebellar Purkinje neurons hyper-excitable and eliminates Ca2+-Na+ spike bursts. Eur J Neurosci (2008) 27(1):93–103. 10.1111/j.1460-9568.2007.05998.x
63.
Shirley TL Rao LM Hess EJ Jinnah HA . Paroxysmal dyskinesias in mice. Mov Disord (2008) 23(2):259–64. 10.1002/mds.21829
64.
Neychev VK Fan X Mitev VI Hess EJ Jinnah HA . The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain (2008) 131(Pt 9):2499–509. 10.1093/brain/awn168
65.
LeDoux MS Lorden JF Meinzen-Derr J . Selective elimination of cerebellar output in the genetically dystonic rat. Brain Res (1995) 697(1-2):91–103. 10.1016/0006-8993(95)00792-o
66.
Yokoi F Dang MT Li Y . Improved motor performance in Dyt1 ΔGAG heterozygous knock-in mice by cerebellar Purkinje-cell specific Dyt1 conditional knocking-out. Behav Brain Res (2012) 230(2):389–98. 10.1016/j.bbr.2012.02.029
67.
LeDoux MS Lorden JF Ervin JM . Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Exp Neurol (1993) 120(2):302–10. 10.1006/exnr.1993.1064
68.
Levy A Lang AE . Ataxia-telangiectasia: a review of movement disorders, clinical features, and genotype correlations. Mov Disord (2018) 33(8):1238–47. 10.1002/mds.27319
69.
Kim M Kim AR Park J Kim JS Ahn JH Park WY et al Clinical characteristics of ataxia-telangiectasia presenting dystonia as a main manifestation. Clin Neurol Neurosurg (2020) 199:106267. 10.1016/j.clineuro.2020.106267
70.
Kojovic M Pareés I Kassavetis P Palomar FJ Mir P Teo JT et al Secondary and primary dystonia: pathophysiological differences. Brain (2013) 136(Pt 7):2038–49. 10.1093/brain/awt150
71.
Bostan AC Dum RP Strick PL . The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A (2010) 107(18):8452–6. 10.1073/pnas.1000496107
72.
Chen CH Fremont R Arteaga-Bracho EE Khodakhah K . Short latency cerebellar modulation of the basal ganglia. Nat Neurosci (2014) 17(12):1767–75. 10.1038/nn.3868
73.
Bostan AC Strick PL . The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci (2018) 19(6):338–50. 10.1038/s41583-018-0002-7
74.
Schrock LE Ostrem JL Turner RS Shimamoto SA Starr PA . The subthalamic nucleus in primary dystonia: single-unit discharge characteristics. J Neurophysiol (2009) 102(6):3740–52. 10.1152/jn.00544.2009
75.
Klier EM Wang H Constantin AG Crawford JD . Midbrain control of three-dimensional head orientation. Science (2002) 295(5558):1314–6. 10.1126/science.1067300
76.
Shaikh AG Zee DS Crawford JD Jinnah HA . Cervical dystonia: a neural integrator disorder. Brain (2016) 139(Pt 10):2590–9. 10.1093/brain/aww141
77.
Shaikh AG Meng H Angelaki DE . Multiple reference frames for motion in the primate cerebellum. J Neurosci (2004) 24(19):4491–7. 10.1523/JNEUROSCI.0109-04.2004
78.
Corp DT Joutsa J Darby RR Delnooz CCS van de Warrenburg BPC Cooke D et al Network localization of cervical dystonia based on causal brain lesions. Brain (2019) 142(6):1660–74. 10.1093/brain/awz112
79.
Delmas-Marsalet P Van Bogaert L . Sur un cas de myoclonies rythmiques continues déterminées par une intervention chirurgicale sur le tronc cérébral. Rev Neurol (1935) 64:728–40.
80.
Cooper IS Upton AR . Use of chronic cerebellar stimulation for disorders of disinhibition. Lancet. (1978) 1(8064):595–600. 10.1016/s0140-6736(78)91038-3
81.
Sokal P Rudas M Harat M Szylberg L Zielinski P . Deep anterior cerebellar stimulation reduces symptoms of secondary dystonia in patients with cerebral palsy treated due to spasticity. Clin Neurol Neurosurg (2015) 135:62–8. 10.1016/j.clineuro.2015.05.017
82.
Beckinghausen J Donofrio SG Lin T Miterko LN White JJ Lackey EP et al Deep brain stimulation of the interposed cerebellar nuclei in a conditional genetic mouse model with dystonia. Adv Neurobiol (2023) 31:93–117. 10.1007/978-3-031-26220-3_6
83.
Salazar Leon LE Kim LH Sillitoe RV . Cerebellar deep brain stimulation as a dual-function therapeutic for restoring movement and sleep in dystonic mice. Neurotherapeutics (2024) 21(6):e00467. 10.1016/j.neurot.2024.e00467
84.
Horisawa S Arai T Suzuki N Kawamata T Taira T . The striking effects of deep cerebellar stimulation on generalized fixed dystonia: case report. J Neurosurg (2019) 132(3):712–6. 10.3171/2018.11.JNS182180
85.
Horisawa S Kohara K Nonaka T Mochizuki T Kawamata T Taira T . Case report: deep cerebellar stimulation for tremor and dystonia. Front Neurol (2021) 12:642904. 10.3389/fneur.2021.642904
86.
Brown EG Bledsoe IO Luthra NS Miocinovic S Starr PA Ostrem JL . Cerebellar deep brain stimulation for acquired hemidystonia. Mov Disord Clin Pract (2020) 7(2):188–93. 10.1002/mdc3.12876
87.
Cajigas I Morrison MA Luciano MS Starr PA . Cerebellar deep brain stimulation for the treatment of movement disorders in cerebral palsy. J Neurosurg (2023) 139(3):605–14. 10.3171/2023.1.JNS222289
88.
Lin S Zhang C Li H Wang Y Wu Y Wang T et al High frequency deep brain stimulation of superior cerebellar peduncles in a patient with cerebral palsy. Tremor Other Hyperkinet Mov (N Y) (2020) 10:38. 10.5334/tohm.551
89.
Lin S Li N Shu Y Wang T Huang P Pan Y et al Superior cerebellar peduncle deep brain stimulation for cerebral palsy. J Neurosurg (2024) 141(5):1407–17. 10.3171/2024.2.JNS232471
90.
Stroud A Tisch S Jonker BP . Cerebellar cortex stimulation for acquired dystonia: a case report and review of its role in modern surgical practice. Stereotact Funct Neurosurg (2022) 100(5-6):321–30. 10.1159/000526072
91.
San Luciano M Oehrn CR Wang SS Tolmie JS Wiltshire A Graff RE et al Protocol for combined N-of-1 trials to assess cerebellar neurostimulation for movement disorders in children and young adults with dyskinetic cerebral palsy. BMC Neurol (2024) 24(1):145. 10.1186/s12883-024-03633-z
92.
Koch G Porcacchia P Ponzo V Carrillo F Cáceres-Redondo MT Brusa L et al Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul (2014) 7(4):564–72. 10.1016/j.brs.2014.05.002
93.
Verhagen MM Martin JJ van Deuren M Ceuterick-de Groote C Weemaes CM Kremer BH et al Neuropathology in classical and variant ataxia-telangiectasia. Neuropathology (2012) 32(3):234–44. 10.1111/j.1440-1789.2011.01263.x
94.
Kuhm C Gallenmuller C Dork T Menzel M Biskup S Klopstock T . Novel ATM mutation in a German patient presenting as generalized dystonia without classical signs of ataxia-telangiectasia. J Neurol (2015) 262(3):768–70. 10.1007/s00415-015-7636-4
95.
Meneret A Ahmar-Beaugendre Y Rieunier G Mahlaoui N Gaymard B Apartis E et al The pleiotropic movement disorders phenotype of adult ataxia-telangiectasia. Neurology (2014) 83(12):1087–95. 10.1212/WNL.0000000000000794
96.
Xiao J Ledoux MS . Caytaxin deficiency causes generalized dystonia in rats. Brain Res Mol Brain Res (2005) 141(2):181–92. 10.1016/j.molbrainres.2005.09.009
97.
White JJ Sillitoe RV . Genetic silencing of olivocerebellar synapses causes dystonia-like behaviour in mice. Nat Commun (2017) 8:14912. 10.1038/ncomms14912
Summary
Keywords
dystonia, cerebellum, deep brain stimulation, dystonia network, animal model
Citation
Wang AS, Alkhodair IM and Kilbane CW (2025) The role of the cerebellum in dystonia. Dystonia 4:14692. doi: 10.3389/dyst.2025.14692
Received
27 March 2025
Accepted
06 June 2025
Published
27 June 2025
Volume
4 - 2025
Edited by
Roy Sillitoe, Baylor College of Medicine, United States
Updates
Copyright
© 2025 Wang, Alkhodair and Kilbane.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander S. Wang, alexander.wang@uhhospitals.org
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.